InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global PARP Inhibitor Biomarkers Market – (By Product (Kits, Assays), By Services (BRCA 1 & 2 Testing, HRD Testing, HRR Testing, Others), By Application (Breast Cancer, Ovarian Cancer, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031.”
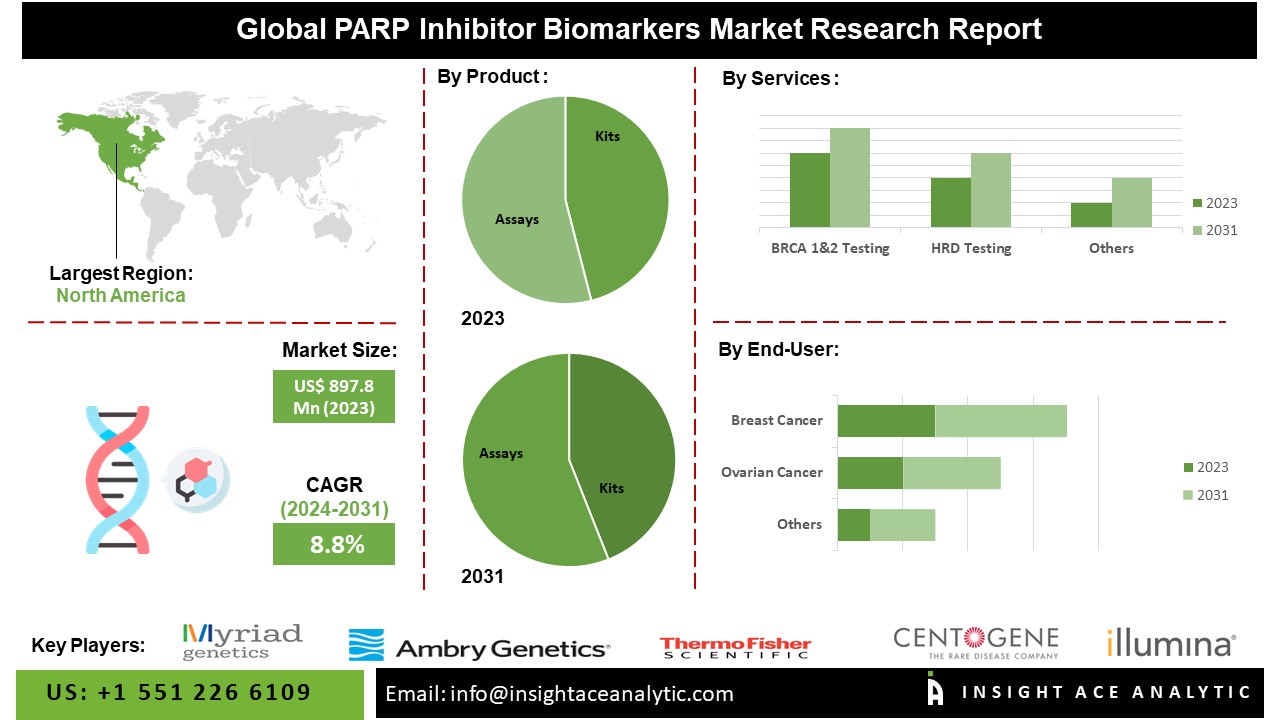
According to the latest research by InsightAce Analytic, the Global PARP Inhibitor Biomarkers Market is valued at US$ 897.8 Billion in 2023, and it is expected to reach US$ 1,751.6 Billion by 2031, with a CAGR of 8.8 % during the forecast period of 2024-2031.
The PARP Inhibitor Biomarkers Market encompasses a comprehensive landscape of biomarkers associated with PARP (Poly ADP-ribose polymerase) inhibitors, which are vital in cancer treatment. These biomarkers are pivotal in predicting patient response to PARP inhibitor therapy, facilitating treatment decisions, and monitoring treatment efficacy. Through genetic mutations, protein expression levels, and other molecular characteristics, these biomarkers offer insights into patient sensitivity to PARP inhibitors, with BRCA1/2 mutations notably linked to heightened sensitivity. In clinical practice, PARP inhibitor biomarkers are utilized for patient selection, treatment stratification, and treatment response monitoring, thereby enabling personalized approaches to therapy. Technological advancements, particularly in genomic sequencing, have enhanced the identification and characterization of these biomarkers, fostering precision medicine in cancer treatment. Key trends in the market comprise the rising adoption of biomarker-guided therapy selection and expanding research endeavours to identify novel biomarkers. The market is served by a diverse array of companies and research institutions involved in biomarker discovery, development, and commercialization, collectively advancing personalized cancer treatment through the optimization of PARP inhibitor therapy.
Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/2447
List of Prominent Players in the PARP Inhibitor Biomarkers Market:
- Myriad Genetics, Inc.
- Ambry Genetics
- Thermo Fisher Scientific, Inc.
- Illumina, Inc.
- CENTOGENE N.V.
- Amoy Diagnostics Co., Ltd.
- Invitae Corporation
- NeoGenomics Laboratories.
- QIAGEN
- Agilent Technologies, Inc.
- F. Hoffmann-La Roche AG
- Bio Rad Laboratories Inc.,
- Exagen Inc.,
- Genway Biotech, Inc.,
- Svar Life Science AB
- Siemens Healthcare GmbH,
- Other Market Players
Market Dynamics:
Drivers-
The PARP Inhibitor Biomarkers Market is propelled by a myriad of factors contributing to its growth trajectory. Firstly, the escalating incidence of cancer, notably breast and ovarian cancer, underscores the crucial role of PARP inhibitors in treatment regimens, fueling the demand for biomarkers to predict patient response and tailor therapies accordingly. Moreover, the paradigm shift towards precision medicine accentuates the importance of biomarker-guided treatments, optimizing patient outcomes while minimizing unnecessary costs. As the applications of PARP inhibitors extend beyond traditional cancer types, such as prostate, pancreatic, and lung cancer, the need for biomarkers to identify responsive patient populations escalates correspondingly. Concurrently, ongoing clinical trials and research initiatives are instrumental in identifying novel biomarkers and enhancing our understanding of PARP inhibitor response. Regulatory approvals and favourable reimbursement policies further bolster the adoption of PARP inhibitors, with biomarkers playing a pivotal role in supporting regulatory submissions and reimbursement decisions. Technological advancements in genomics and molecular diagnostics enable comprehensive tumour profiling, facilitating biomarker discovery and validation.
Challenges:
The PARP Inhibitor Biomarkers Market faces several restraints that may hinder its growth and adoption. The complexity and variability of cancer biology present challenges in identifying reliable and predictive biomarkers for PARP inhibitor therapy. Cancer is a heterogeneous disease, and biomarkers that are effective in one type of cancer may not necessarily translate to others. Additionally, the need for standardized methods for biomarker assessment and interpretation across different laboratories and clinical settings poses a challenge to the consistent and accurate evaluation of PARP inhibitor biomarkers. Moreover, the high cost associated with biomarker testing and the need for specialized equipment and expertise can limit accessibility, particularly in resource-constrained healthcare systems or regions. This may restrict the widespread adoption of PARP inhibitor biomarker testing, particularly in low- and middle-income countries.
Regional Trends:
The North American PARP Inhibitor Biomarkers Market is expected to register a major market share. North America boasts an advanced healthcare infrastructure comprising research institutions and pharmaceutical companies. These resources facilitate the development, testing, and adoption of PARP inhibitor biomarkers, thereby driving market expansion. Secondly, the region grapples with a notably high incidence of cancer, making PARP inhibitors a promising therapeutic avenue, particularly in patients with BRCA mutations. Besides, the APAC region had a substantial share of the market. Leads in biomedical research and innovation, with pharmaceutical companies and research institutions actively engaged in pioneering new biomarkers for various diseases, including cancer. This robust research and development landscape significantly contributes to the proliferation of the region’s PARP inhibitor biomarkers market.
Curious About This Latest Version Of The Report? Enquiry Before Buying: https://www.insightaceanalytic.com/report/parp-inhibitor-biomarkers-market/2447
Recent Developments:
- In April 2024, Bio-Rad Laboratories, Inc. introduced its inaugural ultrasensitive multiplexed digital PCR assay, known as the ddPLEX ESR1 Mutation Detection Kit. The assay enhanced the company’s Droplet Digital PCR (ddPCR™) product line for the field of oncology. These mutation detection assays are very sensitive and capable of detecting several mutations simultaneously. They are valuable for translational research, selecting appropriate therapies, and monitoring disease progression.
- In February 2024, Myriad Genetics, Inc., a renowned genetic testing and precision medicine firm, successfully finalized the acquisition of specific assets from Intermountain Health’s Intermountain Precision Genomics (IPG) laboratory business. This acquisition encompasses Precision Oncology Testing, Precision Fluid Testing, and their CLIA-certified laboratory based in St. George, Utah.
Segmentation of PARP Inhibitor Biomarkers Market-
By Product
- Kits
- Assays
By Services
- BRCA 1 & 2 Testing
- HRD Testing
- HRR Testing
- Others
By Application
- Breast Cancer
- Ovarian Cancer
- Others
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- South East Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/2447
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/