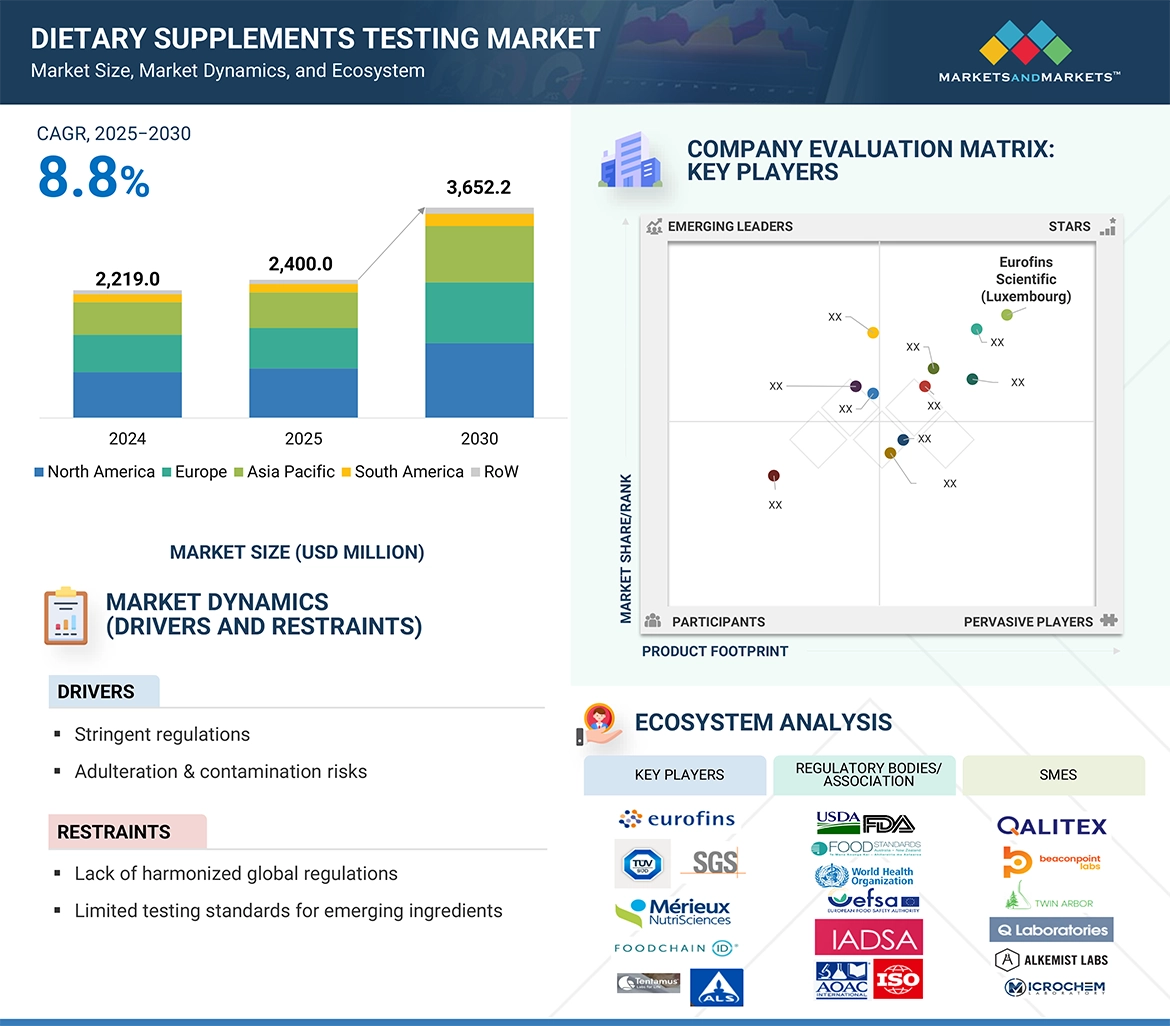
The dietary supplements testing market is estimated at USD 2,400.0 million in 2025 and is projected to reach USD 3,652.2 million, at a CAGR of 8.8% from 2025 to 2030. The demand for dietary supplement testing is expected to rise significantly, driven by increasing consumer awareness about product safety, quality, and regulatory compliance, as well as the growing demand for global dietary supplement products. Testing services, encompassing potency, identity/authentication, contaminants, microbiological, adulteration, stability, allergen, GMO, label claim, and functional claims testing, provide reliable verification of product efficacy and safety. These services are essential for ensuring that supplements meet regulatory standards and deliver scientifically validated health benefits. With the growing preference for clean-label, high-quality, and functional products, manufacturers are increasingly relying on advanced testing services to maintain consumer trust, minimize risks, and support the development of innovative supplement formulations across the nutraceutical and health supplement sectors.
Dietary Supplements Testing Market Driver: Stringent regulations
The global dietary supplements industry is governed by a complex web of regulatory frameworks aimed at ensuring product safety, efficacy, and transparency. Regulatory bodies such as the US Food and Drug Administration (FDA), European Food Safety Authority (EFSA), and agencies under Codex Alimentarius mandate strict guidelines for manufacturing, labelling, and claims. In the US, the Dietary Supplement Health and Education Act (DSHEA) of 1994 classifies supplements as a category of food, but manufacturers must still ensure that products are not adulterated and meet current Good Manufacturing Practices (cGMP). This regulatory pressure fuels the need for robust testing services to verify ingredient identity, purity, and potency, especially in multi-ingredient products.
Recent developments by the United States Pharmacopeia (USP) underscore this growing demand for quality assurance. The draft of General Chapter addresses the complexity of multi-ingredient dietary supplements and introduces a more harmonized approach to quality testing. It emphasizes the need for scientifically validated methods to prevent cross-contamination, mislabeling, and adulteration, issues that have led to widespread recalls and legal scrutiny in the past. Such evolving standards not only elevate consumer trust but also necessitate advanced analytical capabilities from third-party testing labs. Moreover, as dietary supplement usage expands, particularly in regions such as North America, Asia Pacific, and Europe, governments are increasingly implementing stricter compliance frameworks to monitor product quality. In response, regulatory agencies are expanding surveillance and enforcement, while consumers are becoming more informed and demanding evidence-backed products. Together, these dynamics are propelling the demand for dietary supplement testing as both a regulatory requirement and a market differentiator.
Traditional testing services segment to hold significant market share during forecast period
In the dietary supplements testing market, traditional testing services are expected to hold a significant market share during the forecast period, driven by their widespread adoption, reliability, and regulatory recognition. These services include conventional analytical methods such as wet chemistry, microbiological assays, and standard chromatography, which remain the preferred choice for routine quality assurance and regulatory compliance.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=227592621
Functional claims testing to be fastest-growing test type segment during the forecast period
The rising demand for scientifically validated health benefits in dietary supplements is fueling growth in functional claims testing, which is projected to be the fastest-growing test type segment in the market during the forecast period. Consumers increasingly seek products that deliver verified outcomes, such as immunity support, digestive health, cognitive benefits, and overall wellness, making functional claims testing essential for substantiating label claims and ensuring regulatory compliance. These testing services enable manufacturers to demonstrate efficacy and safety, building consumer trust and supporting marketing claims. Coupled with other critical analyses such as potency, identity/authentication, contaminants, microbiological, stability, allergen, and GMO testing, functional claims testing helps dietary supplement companies offer innovative, reliable, and high-quality products. As the global dietary supplements industry expands and consumer interest in evidence-backed, health-oriented products grows, functional claims testing is poised to play a central role in ensuring product credibility and driving market adoption.
Asia Pacific to be fastest-growing market during forecast period.
The Asia Pacific region is projected to be the fastest-growing market for dietary supplements testing during the forecast period, driven by increasing health awareness, consumer focus on health supplement products, and growing consumption of dietary supplements across countries such as China, India, Japan, and Australia. Rapidly expanding supplement markets in the region are creating a strong demand for reliable testing services to ensure product safety, potency, and regulatory compliance. Active participation by regional industry players and collaborations, such as partnerships between testing laboratories and local supplement manufacturers, are facilitating the development of innovative testing solutions that streamline verification processes and improve market accessibility. These initiatives enhance the credibility of dietary supplements, ensure adherence to international quality standards, and enable manufacturers to meet the evolving expectations of health-conscious consumers, thereby driving market growth across the Asia Pacific.
The report profiles key players such as Eurofins Scientific (Luxembourg), SGS Société Générale de Surveillance SA (Switzerland), Intertek Group plc (UK), Mérieux NutriSciences (US), UL Solutions (US), TÜV SÜD (Germany), ALS (Australia), Tentamus (Italy), Agrolab Group (Germany), FoodChain ID (US), Certified Laboratories (US), Element Materials Technology (UK), NSF (US), Vimta Labs Ltd. (India), Qalitex (US), Alkemist (US), and Anresco Laboratories (US).
Recent Developments in the Dietary Supplements Testing Industry:
In July 2025, Mérieux NutriSciences finalized its acquisition of Bureau Veritas’ food testing operations in Ecuador, marking its entry into the Ecuadorian market and expanding its footprint across Latin America. The move integrates a 750 sq meter laboratory and over 80 employees into Mérieux’s global network of 140+ ISO 17025-accredited labs. This strategic acquisition enhances capabilities in microbiological, nutritional, chemical, and molecular testing, aiming to deliver innovative, science-based solutions to support Ecuador’s export-driven food industry.
In March 2025, SGS SA entered into a strategic partnership with Nutralong, a biotechnology company specializing in dietary supplements, to establish a joint laboratory. This collaboration enhances SGS’s testing and validation capabilities in the nutraceutical sector, aligning with global standards and expanding its footprint in China. The joint lab leverages SGS’s advanced equipment and testing frameworks to improve ingredient accuracy, batch traceability, and regulatory compliance across key markets like the EU and US, thereby strengthening supply chain security and customer trust.
In October 2024, SGS North America expanded its food and nutraceutical testing capabilities by relocating to a larger, state-of-the-art facility in Fairfield, New Jersey. This move enhances its ability to provide rapid and traditional pathogen testing, environmental monitoring, and quality assurance services for food, pet food, and wellness products. The expansion responds to growing demand driven by stricter food safety regulations and consumer expectations for high-quality products. The new facility complements SGS’s existing network of ISO/IEC 17025-accredited labs across North America.
Request Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=227592621
About MarketsandMarkets™
MarketsandMarkets™ has been recognized as one of America’s Best Management Consulting Firms by Forbes, as per their recent report.
MarketsandMarkets™ is a blue ocean alternative in growth consulting and program management, leveraging a man-machine offering to drive supernormal growth for progressive organizations in the B2B space. With the widest lens on emerging technologies, we are proficient in co-creating supernormal growth for clients across the globe.
Today, 80% of Fortune 2000 companies rely on MarketsandMarkets, and 90 of the top 100 companies in each sector trust us to accelerate their revenue growth. With a global clientele of over 13,000 organizations, we help businesses thrive in a disruptive ecosystem.
The B2B economy is witnessing the emergence of $25 trillion in new revenue streams that are replacing existing ones within this decade. We work with clients on growth programs, helping them monetize this $25 trillion opportunity through our service lines – TAM Expansion, Go-to-Market (GTM) Strategy to Execution, Market Share Gain, Account Enablement, and Thought Leadership Marketing.
Built on the ‘GIVE Growth’ principle, we collaborate with several Forbes Global 2000 B2B companies to keep them future-ready. Our insights and strategies are powered by industry experts, cutting-edge AI, and our Market Intelligence Cloud, KnowledgeStore™, which integrates research and provides ecosystem-wide visibility into revenue shifts.
Media Contact
Company Name: MarketsandMarkets™ Research Private Ltd.
Contact Person: Mr. Rohan Salgarkar
Email: Send Email
Phone: 18886006441
Address:1615 South Congress Ave. Suite 103, Delray Beach, FL 33445
City: Florida
State: Florida
Country: United States
Website: https://www.marketsandmarkets.com/Market-Reports/dietary-supplements-testing-market-227592621.html

