DelveInsight’s ‘Wet AMD Pipeline Insight 2022’ report provides comprehensive global coverage of available, marketed, and pipeline Wet AMD therapies in various stages of clinical development, major pharmaceutical players working to advance the pipeline space and future growth potential of the Wet AMD pipeline domain.
Key Takeaways from the Wet AMD Pipeline Report
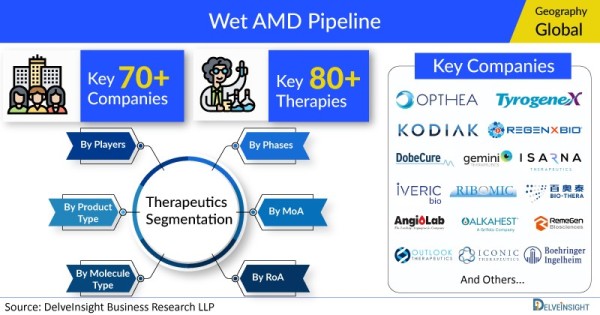
- DelveInsight’s Wet AMD pipeline analysis depicts a robust space with 70+ active players working to develop 80+ pipeline therapies.
- Some of the key pharmaceutical players working to develop potential Wet AMD drug candidates to improve the Wet AMD treatment landscape include Opthea Limited, Outlook Therapeutics, Kodiak Sciences, Regenxbio, Dobecure, Gemini Therapeutics, Huabo Biopharm, Isarna Therapeutics, IVERIC bio (formerly Ophthotech Corporation), Ribomic, Curacle, Bio-Thera Solutions, AngioLab Inc, Alkahest, Tyrogenex, Iconic Therapeutics, AiViva BioPharma, Boehringer Ingelheim, RemeGen, PanOptica, Clearside Biomedical, Feramda, AsclepiX Therapeutics, Unity Biotechnology, Kyowa Kirin, Ocular Therapeutix, Janssen Research & Development, EyePoint Pharmaceuticals, Shanghai Biomabs Pharmaceutical, Aerie Pharmaceuticals, Ashvattha Therapeutics, Roche, Sunshine Guojian Pharmaceutical (Shanghai), Adverum Biotechnologies, TOT Biopharm, 4D Molecular Therapeutics, Abpro Corporation, Novelty Nobility, Kala Pharmaceuticals, Surrozen, Eyevensys, Ocugen, Exonate, and others.
- Key Wet AMD pipeline therapies in various stages of development include OPT-302, ONS-5010, KSI-301, RGX-314, Etamsylate, GEM-103, HB002.1M, ISTH0036, Zimura, RBM-007, CU03, PF-04523655, BAT5906, ALS-L1023, AKST4290, Vorolanib (X-82), ICON-1, AIV007, BI 836880, RC 28 E, PAN 90806, CLS-AX, AS101, AXT107, UBX1325, KHK4951, OTX-TKI, AAVCAGsCD59, EYP-1901, CMAB818, AR-13503, D-4517, RG6120, 601A, ADVM-022, TAB014, 4D-150, ABP-201, NN2101, EYS609, OCU 200, EXN 169, and others.
- In August 2022, Opthea Limited announced a non-dilutive financing transaction for up to US$170 million from investment funds working with Launch Therapeutics (Launch Tx) to finance and advance the ongoing Phase 3 clinical trials and pre-commercialization activities of OPT-302 for wet age-related macular degeneration (wet AMD).
- In August 2022, Outlook Therapeutics, Inc. announced that it had re-submitted its Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for ONS-5010, an investigational ophthalmic therapy which, if approved, will be branded as LYTENAVA™ (bevacizumab-vikg) for the treatment of wet age-related macular degeneration (wet AMD).
- In August 2022, EyePoint Pharmaceuticals, Inc. announced that the first patient had been dosed in Phase 2 “Durasert® and Vorolanib in Ophthalmology 2” (DAVIO 2) clinical trial of EYP-1901, an investigational sustained delivery anti-vascular endothelial growth factor (anti-VEGF) treatment for wet age-related macular degeneration (wet AMD).
- In August 2022, Alcon and Aerie Pharmaceuticals, Inc. Announced the companies had entered into a definitive merger agreement through which Alcon would acquire Aerie.
- In July 2022, REGENXBIO Inc. announced it had completed enrollment in Cohort 5 of the Phase II AAVIATE® trial of RGX-314 for the treatment of wet age-related macular degeneration (wet AMD) using in-office suprachoroidal delivery.
- In July 2022, IVERIC bio, Inc. and DelSiTech Ltd, announced an exclusive global license agreement providing Iveric Bio with the right to develop and commercialize new formulations of Zimura® (avacincaptad pegol) using DelSiTech’s silica-based sustained release technology. As part of Iveric Bio’s lifecycle expansion plan for Zimura, the Company is committed to developing sustained release technologies for the treatment of age-related macular degeneration (AMD). These technologies could potentially address patients being treated for geographic atrophy (GA) and intermediate AMD.
- In July 2022, Clearside Biomedical, Inc. announced the completion of dosing in Cohorts 3 and 4 of OASIS, its Phase 1/2a clinical trial of CLS-AX (axitinib injectable suspension) in patients with neovascular age-related macular degeneration (wet AMD).
- In March 2022, RIBOMIC Inc. announced the results from the investigator-sponsored trial (IST), TEMPURA, along with updated data from its TOFU and RAMEN studies with RBM-007, an investigational anti-fibroblast growth factor-2 aptamer, in wet age-related macular degeneration (wAMD).Early signs of efficacy in the TEMPURA study provide initial support of clinical benefit in treatment-naïve wAMD. Further analysis of Phase 2 TOFU data and results from the RAMEN study in previously treated wAMD show no benefit of RBM-007 monotherapy or the combination over Eylea in any of the study outcome measures. The new data suggests RBM-007 could be more effective in treatment-naïve vs previously treated wAMD.
- In December 2021, Gemini Therapeutics received six-month data for the 50 patients enrolled in the wet AMD study. This study was designed to investigate the safety and tolerability of GEM103 as an adjunct to standard of care aflibercept therapy, with patients randomized 2:1 between a GEM103 plus aflibercept arm and a sham comparator plus aflibercept arm. The interim analysis showed that intravitreal GEM103 plus aflibercept was generally well-tolerated, and the safety profile was generally consistent with the sham plus aflibercept arm. Patients in this study were dosed every other month concurrently with aflibercept. CFH levels remained supraphysiologic and greater than five times above baseline at the trough time points throughout the six months.
Request a sample and discover the recent advances in Wet AMD treatment drugs @Wet AMD Pipeline Report
The Wet AMD pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage Wet AMD products, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the Wet AMD pipeline landscape.
Wet AMD Overview
Wet AMD, also known as neovascular AMD or exudative AMD, is a type of retinal degeneration characterized by abnormal choroidal neovascularization beneath the retina and macula lutea. Wet AMD is less common but more severe than dry AMD. The wet/neovascular type affects 10-15% of people with age-related macular degeneration but accounts for 90% of all cases of severe vision loss caused by the disease. It is responsible for 90% of severe vision loss in people with AMD. Everyone who gets wet AMD used to have dry AMD.
Central visual blurring and distortion are the most common Wet AMD symptoms. The majority of patients complain that straight lines look crooked or wavy. Color photographs, fluorescein angiography, and optical coherence tomography can help confirm the Wet AMD diagnosis and guide Wet AMD treatment.
Find out more about the disease and recent developments in Wet AMD drugs @Wet AMD Treatment Drugs
Wet AMD Pipeline Drugs
|
Drug |
Company |
Phase |
MoA |
RoA |
|
LY09004 |
Luye Pharma |
Phase III |
Vascular endothelial growth factor A inhibitor |
Intraocular |
|
KSI-301 |
Kodiak Sciences |
Phase III |
Vascular endothelial growth factors inhibitor |
Intravitreal |
|
GEM-103 |
Gemini Therapeutics |
Phase II |
Complement factor H replacement |
Intravitreal |
|
Zimura |
Iveric Bio |
Phase II |
Complement C5 inhibitor |
Intravitreal |
|
RC 28 E |
RemeGen |
Phase I/II |
Vascular endothelial growth factors inhibitor |
Intravitreal |
|
BI 836880 |
Boehringer Ingelheim |
Phase I/II |
Vascular endothelial growth factor A inhibitor |
Intravitreal |
|
AAVCAGsCD59 |
Janssen Research and Development |
Phase I |
Complement system protein inhibitor |
Intravitreal |
Learn more about the emerging Wet AMD pipeline therapies @Wet AMD Clinical Trials
Wet AMD Therapeutics Assessment
The Wet AMD pipeline report proffers an integral view of the Wet AMD emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Wet AMD Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III, Pre-registration
- Therapeutics Assessment By Route of Administration: Inhalation, Intranasal, Intravenous, Oral, Parenteral, Subcutaneous
- Therapeutics Assessment By Molecule Type: Antibody, Antisense oligonucleotides, Immunotherapy, Monoclonal antibody, Peptides, Protein, Recombinant protein, Small molecule, Stem cell, Vaccine
- Therapeutics Assessment By Mechanism of Action: Placenta growth factor inhibitors, Vascular endothelial growth factor A inhibitors, Vascular endothelial growth factors inhibitors, Complement factor H replacements, Complement C5 inhibitors, Fibroblast growth factor inhibitors, Angiopoietin-2 inhibitors, Complement system protein inhibitors, Gene transference
- Key Wet AMD Companies: Opthea Limited, Outlook Therapeutics, Kodiak Sciences, Regenxbio, Dobecure, Gemini Therapeutics, Huabo Biopharm, Isarna Therapeutics, IVERIC bio (formerly Ophthotech Corporation), Ribomic, Curacle, Bio-Thera Solutions, AngioLab Inc, Alkahest, Tyrogenex, Iconic Therapeutics, AiViva BioPharma, Boehringer Ingelheim, RemeGen, PanOptica, Clearside Biomedical, Feramda, AsclepiX Therapeutics, Unity Biotechnology, Kyowa Kirin, Ocular Therapeutix, Janssen Research & Development, EyePoint Pharmaceuticals, Shanghai Biomabs Pharmaceutical, Aerie Pharmaceuticals, Ashvattha Therapeutics, Roche, Sunshine Guojian Pharmaceutical (Shanghai), Adverum Biotechnologies, TOT Biopharm, 4D Molecular Therapeutics, Abpro Corporation, Novelty Nobility, Kala Pharmaceuticals, Surrozen, Eyevensys, Ocugen, Exonate, and others.
- Key Wet AMD Pipeline Therapies: OPT-302, ONS-5010, KSI-301, RGX-314, Etamsylate, GEM-103, HB002.1M, ISTH0036, Zimura, RBM-007, CU03, PF-04523655, BAT5906, ALS-L1023, AKST4290, Vorolanib (X-82), ICON-1, AIV007, BI 836880, RC 28 E, PAN 90806, CLS-AX, AS101, AXT107, UBX1325, KHK4951, OTX-TKI, AAVCAGsCD59, EYP-1901, CMAB818, AR-13503, D-4517, RG6120, 601A, ADVM-022, TAB014, 4D-150, ABP-201, NN2101, EYS609, OCU 200, EXN 169, and others.
Dive deep into rich insights for drugs for Wet AMD treatment, visit @Wet AMD Treatment
Table of Contents
|
1. |
Wet AMD Pipeline Report Introduction |
|
2. |
Wet AMD Pipeline Report Executive Summary |
|
3. |
Wet AMD Pipeline: Overview |
|
4. |
Analytical Perspective In-depth Commercial Assessment |
|
5. |
Wet AMD Pipeline Therapeutics |
|
6. |
Wet AMD Pipeline: Late Stage Products (Pre-registration) |
|
7. |
Wet AMD Pipeline: Late Stage Products (Phase III) |
|
8. |
Wet AMD Pipeline: Mid Stage Products (Phase II) |
|
9. |
Wet AMD Pipeline: Early Stage Products (Phase I) |
|
10. |
Wet AMD Pipeline Therapeutics Assessment |
|
11. |
Inactive Products in the Wet AMD Pipeline |
|
12. |
Company-University Collaborations (Licensing/Partnering) Analysis |
|
13. |
Key Companies |
|
14. |
Key Products in the Wet AMD Pipeline |
|
15. |
Unmet Needs |
|
16. |
Market Drivers and Barriers |
|
17. |
Future Perspectives and Conclusion |
|
18. |
Analyst Views |
|
19. |
Appendix |
For further information on Wet AMD pipeline therapeutics, reach out @Wet AMD Drugs
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email: Send Email
Phone: +19193216187
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/report-store/wet-age-related-macular-degeneration-wet-amd-pipeline-insight

