InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global TROP2 (Trophoblast Cell-surface Antigen 2) Targeted Cancer Therapy Market- (By Application (Breast Cancer, Prostate Cancer, Lung Cancer, Colorectal Cancer, Pancreatic Cancer, and Others)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031.”
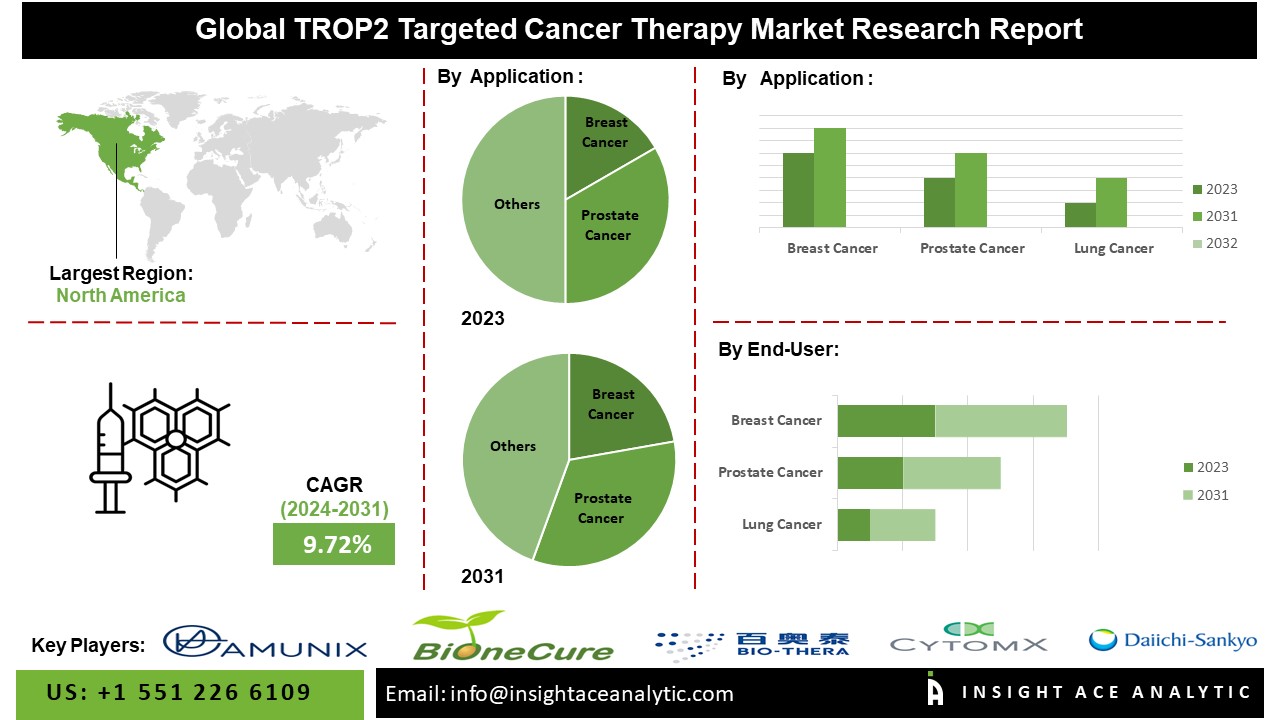
According to the latest research by InsightAce Analytic, the Global TROP2 (Trophoblast Cell-surface Antigen 2) Targeted Cancer Therapy Market is expected to increase with a CAGR of 9.72% during the forecast period of 2024-2031.
Market Synopsis-
The TROP2 (trophoblast cell-surface antigen targeted cancer therapy market encompasses pharmaceuticals and biotechnological products designed to target cancer cells expressing the TROP2 protein specifically. Several factors drive the market for TROP2-targeted cancer therapy. First, the increasing incidence of cancer worldwide, coupled with the need for more precise and personalized treatment options, fuels the demand for targeted therapies like TROP2 inhibitors or monoclonal antibodies. Additionally, advancements in biotechnology and drug development have led to the discovery and development of novel TROP2-targeted agents with improved efficacy and safety profiles.
Furthermore, the growing understanding of the molecular mechanisms underlying cancer progression and metastasis has highlighted the importance of TROP2 as a therapeutic target. Clinical studies evaluating TROP2-targeted therapies have shown promising results, further driving interest and investment in this area. However, challenges such as drug resistance, limited efficacy in certain cancer types, and regulatory hurdles may hinder the market growth of TROP2-targeted cancer therapy. Additionally, the high cost of novel biotechnological therapies and the need for extensive clinical trials to demonstrate safety and efficacy pose challenges to market adoption.
Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/2493
List of Prominent Players in the TROP2 (trophoblast cell-surface antigen 2) targeted cancer therapy Market:
- Amunix
- BiOneCure Therapeutics
- Bio-Thera Solutions
- CytomX Therapeutics
- Daiichi Sankyo Company
- Eucure Biopharma
- Gilead Sciences
- Janux Therapeutics
- KAEDI Biotech
- Klus Pharma
Market Dynamics:
Drivers:
The rising incidence of TROP2-positive cancers, such as triple-negative breast cancer, lung cancer, and pancreatic cancer, has highlighted the urgent need for effective treatment options. Given the limited efficacy of current standard therapies in these aggressive cancer types, there is a strong demand for targeted therapies that can selectively eliminate TROP2-expressing tumor cells while sparing normal tissues. Moreover, the encouraging clinical trial results demonstrating the potential of TROP2-targeted therapies in improving patient outcomes have fueled investor interest and financial support for further development and commercialization efforts. This includes accelerated regulatory pathways and expedited approvals for promising TROP2-targeted agents, facilitating their entry into the market and expanding treatment options for cancer patients.
Challenges:
There’s the challenge of tumor variability, as TROP2 expression levels can differ significantly both between various tumor types and among individual patients, making it difficult to select patients for TROP2-targeted treatments and predict treatment response accurately. Secondly, the development of resistance poses a significant challenge, with cancer cells potentially evolving mechanisms to resist TROP2-targeted therapies, such as through gene mutations or activation of alternative signaling pathways, thereby complicating efforts to maintain treatment efficacy. Additionally, concerns persist regarding adverse effects, as efforts to selectively target TROP2-expressing cancer cells may inadvertently lead to off-target effects and toxicity in normal tissues expressing low levels of TROP2, necessitating careful management to minimize side effects. Furthermore, the complexity of designing clinical trials for TROP2-targeted therapies, given tumor heterogeneity and the need for personalized treatment approaches, adds another layer of challenge to the field. Regulatory hurdles, such as the stringent requirements for safety and efficacy data for obtaining approval, can prolong the approval process and increase development costs.
Regional Trends:
In North America, particularly in the United States, the market for TROP2-targeted cancer therapies is driven by robust research and development activities, strong regulatory frameworks, and advanced healthcare infrastructure. Clinical trials evaluating the efficacy and safety of TROP2-targeted agents are often initiated and conducted in North America, leveraging the region’s expertise in translational research and clinical trial management. Additionally, the presence of leading pharmaceutical companies and academic institutions focused on oncology research further accelerates the advancement of TROP2-targeted therapies in the North American market. In the Asia-Pacific region, including countries like China, Japan, and South Korea, the TROP2-targeted cancer therapy market is characterized by a rapidly growing healthcare sector, increasing investment in biomedical research, and a large patient population with unmet medical needs. While the regulatory landscape in APAC may vary across countries, there is a growing emphasis on expediting drug approval processes and fostering innovation in cancer treatment.
Enquiry Before Buying: https://www.insightaceanalytic.com/request-sample/2493
Recent Developments:
- In March 2023, Bio-Thera Solutions, Ltd. announced the initiation of dosing in a Phase 1 clinical trial evaluating BAT8008, an antibody-drug conjugate (ADC) designed to target TROP2. This multicenter, open-label Phase 1 study seeks to assess the safety and tolerability of BAT8008 in patients diagnosed with advanced solid tumors.
Segmentation of TROP2 (trophoblast cell-surface antigen 2) targeted cancer therapy Market-
TROP2 (Trophoblast Cell-surface Antigen 2) Targeted Cancer Therapy Market- By Applications
- Breast Cancer
- Prostate Cancer
- Lung Cancer
- Colorectal Cancer
- Pancreatic Cancer
- Others
TROP2 (Trophoblast Cell-surface Antigen 2) Targeted Cancer Therapy Market- By Region
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- South East Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
For More Customization: https://www.insightaceanalytic.com/customisation/2493
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/