Award winning market research company has published a report on the global respiratory virus vaccines market. As per the study, R&D on respiratory vaccines is focused on COVID-19 at the moment, however, the long-term outlook remains positive. Learning from the impact of COVID-19, governments around the globe are focusing on developing adequate treatment options for respiratory vaccines.
With over 110 million active cases globally, pharmaceutical companies are racing against time to introduce potential vaccine candidates. Breakthroughs have manifested themselves in the form of three final products: the Pfizer-BioNTech COVID-19 vaccine, COVAXINTM and Covishield. While inoculation procedures are underway across Europe and North America, the aforementioned candidates are anticipated to knock on the doors of all continents in the current financial year.
With worldwide supply chain disruptions experienced thraoughout 2020, leading manufacturers are seeking to diversify their supply chain bases. Since the past few decades, procurement from low-cost destinations comprised the primary expansion strategy for leading multinational firms. For instance, in 2020, Japan announced a US$ 2.2 billion fundraising to assist healthcare companies to shift their base away from China, followed by the US which deployed a similar tactic.
Countries such as Thailand, India, Vietnam and other South East Asian countries are the preferred destinations. Such initiatives are expected to assist respiratory virus manufacturers to enhance their research & development capacities in the long-run.
“The rapidly mutating nature of different virus species is leading to the emergence of numerous life-threatening respiratory diseases, with COVID-19 occupying center stage presently. This is prompting healthcare companies to augment their R&D capacities by initiating various clinical trials for new drug and vaccine candidates,” says a Fact.MR analyst.
For more Insights into the Market, Request a Sample of this Report
https://www.factmr.com/connectus/sample?flag=S&rep_id=5199
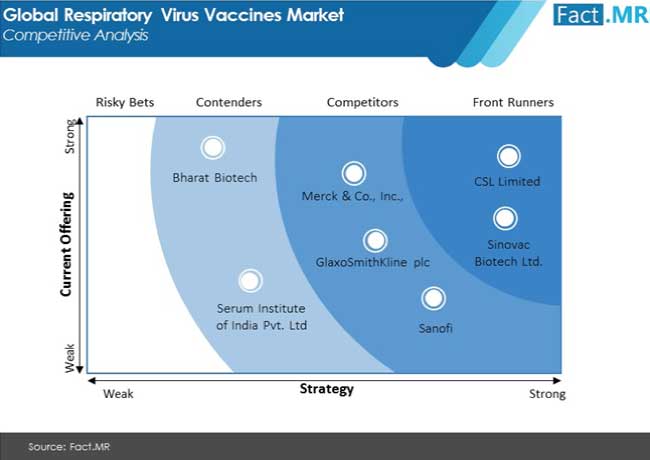
Key Takeaways from Fact.MR’s Respiratory Virus Vaccines Market Study
- By type, inactivated/killed vaccines to experience strong growth through 2021
- Intramuscular vaccine administration expected to remain the preferred mode due to rapid drug absorption
- By indication, COVID-19 vaccinations to remain top priority throughout the current year
- Demand for adult vaccine administration, especially geriatrics, is likely to remain elevated in the short-run
- Hospitals are poised to be the primary respiratory virus vaccine end-users through 2031
- US & UK to experience credible expansion, amid initiation of inoculation procedures amongst frontline health workers and high priority citizens
- Germany emerges as a potential revenue hotspot, with companies such as Pfizer and BioNTech investing in COVID-19 vaccine development
- High incidence of respiratory diseases such as avian influenza and common flu is prompting pharmaceutical companies to invest in India
Competitive Landscape
Prominent respiratory virus vaccine manufacturers profiled by Fact.MR include, but are not limited to, Bharat Biotech, CSL Limited, Sanofi, AstraZeneca, GlaxoSmithKline Plc., Merck & Co. Inc., Serum Institute of India Pvt. Ltd. and Sinovac Biotech Ltd. Continuous research and clinical trials to verify the efficacy of potential vaccine candidates are driving business prospects for the aforementioned providers.
Presently, all manufacturers have reoriented their priorities by accelerating research capacities to develop an effective vaccine to eradicate the COVID-19 pandemic. For instance, Bharat Biotech and Ocugen Inc. successfully inked a definitive agreement for commercializing COVAXINTM, an advanced stage whole-virion inactivated COVID-19 vaccine candidate, for the United States market in February 2021.
Likewise, AstraZeneca has developed the Covishield vaccine which was granted the emergency use authorization across the UK in December 2020. The vaccine, formerly known as AZD1222, has been approved for the active immunization of individuals 18 years or older, with two doses recommended within an interval of 4 to 12 weeks.
Get Customization on this Report for Specific Country
https://www.factmr.com/connectus/sample?flag=RC&rep_id=5199
More Valuable Insights on Respiratory Virus Vaccines Market
Fact.MR, in its new offering, presents an unbiased analysis of the global respiratory virus vaccines market. The study divulges essential insights on the respiratory virus vaccines market on the basis of type (inactivated/killed vaccines, live-attenuated vaccines, and recombinant vaccines), route of administration (intramuscular, intranasal, and subcutaneous), indication (influenza, measles, mumps & rubella, and COVID-19), age group (adults and pediatric), and end user (physician offices, hospitals, clinics, and pharmacies/ stores), across major regions of the world (North America, Latin America, Europe, Asia Pacific, and the Middle East & Africa).
Key Questions Covered in the Report
- What is likely to be the future outlook of the respiratory virus vaccines market?
- How will the coronavirus pandemic shape respiratory virus vaccine development programs?
- Why are the US & UK likely to dominate the global respiratory virus vaccines market in the long-run?
- Why are manufacturers preferring inactivated vaccine candidates?
- Which is the most preferred route of administration for respiratory virus vaccines?
- Which key market players currently operate in the global respiratory virus vaccines landscape?
Read Fact.MR Exclusive Article on Emerging Frontiers in the Global Dental Industry
https://www.factmr.com/article/110/emerging-frontiers-in-the-global-dental-industry
About Fact.MR
Market research and consulting agency with a difference! That’s why 80% of Fortune 1,000 companies trust us for making their most critical decisions. We have offices in US and Dublin, whereas our global headquarter is in Dubai. While our experienced consultants employ the latest technologies to extract hard-to-find insights, we believe our USP is the trust clients have on our expertise. Spanning a wide range – from automotive & industry 4.0 to healthcare & retail, our coverage is expansive, but we ensure even the most niche categories are analyzed. Reach out to us with your goals, and we’ll be an able research partner.
Contact:
US Sales Office:
11140 Rockville Pike Suite
400 Rockville,MD 20852 ,
United States
Tel: +1 (628) 251-1583
Corporate Headquarter:
Unit No: AU-01-H Gold Tower (AU),
Plot No: JLT-PH1-I3A,
Jumeirah Lakes Towers,
Dubai,United Arab Emirates
Email: sales@factmr.com
Media Contact
Company Name: Fact.MR
Contact Person: Neha Bhosale
Email: Send Email
Phone: 6282511583
Address:US Sales Office: 11140 Rockville Pike
City: Rockville
State: Maryland
Country: United States
Website: https://www.factmr.com

