According to a report by Grand View Research, Inc.; the global Pharmacovigilance (PV) market is projected to reach USD 10.27 billion by 2025. Rising cases of Adverse Drug Reactions (ADRs) are anticipated to drive the market during the forecast period (2018 to 2025). Rising consumption of combination of drugs owing to increasing cases of chronic disorders can result in ADRs. This factor is anticipated to augment demand for pharmacovigilance services over the forecast period. Growing number of nonprofit organizations such as International Society of Pharmacovigilance (ISoP) to promote benefits of PV services can further impel market growth.
Supportive government initiatives to promote the use of pharmacovigilance services can drive market growth over the forecast period. Increasing use of medicines to treat chronic diseases is likely to account for a huge proportion of overall consumption of drugs in hospital set-ups. This factor is projected to boost demand for medicines among healthcare providers, thereby driving the need for development of novel therapeutics through extensive clinical trials. Moreover, growing R&D activities in major pharmaceutical companies to develop innovative therapeutic drugs can further boost market growth over the forecast period.
Access Full Research Report On Pharmacovigilance (PV) Market Analysis:
www.grandviewresearch.com/industry-analysis/pharmacovigilance-industry
U.S. pharmacovigilance market revenue by clinical trial phase, 2014 – 2025 (USD million)
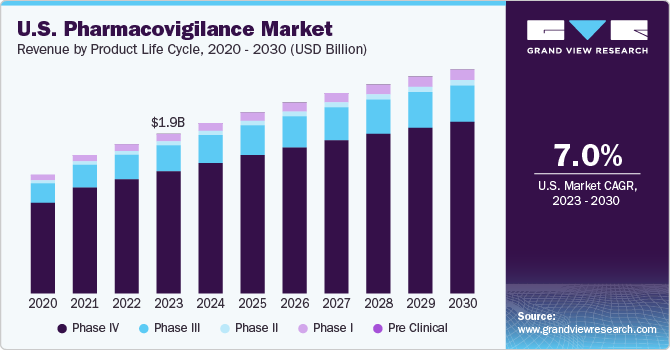
The worldwide Pharmacovigilance (PV) market can be segregated on the basis of clinical trial phase, service provider, type, end-use, and region. Based on clinical trial phase, the market can be categorized into phase 1, phase 2, phase 3, phase 4, and pre-clinical. In 2016, phase 4 segment dominated the market. PV solutions act as additional safety feature for drugs in clinical trials. Phase 4 is estimated to be important stage of clinical trials attributed to detection of unpredicted adverse drug reactions during this stage. Hence, the data monitored and collected during this stage is considered of high relevance.
Based on service provider, the market can be bifurcated into contract outsourcing and in-house. In 2016, contract outsourcing segment dominated the market and is anticipated to expand at the fastest CAGR in the forthcoming years. Benefits associated with PV services including resource flexibility and reduction in fixed cost, risk, and upfront investments are projected to drive the demand over the forecast period.
Browse More Reports Of This Category By Grand View Research At:
www.grandviewresearch.com/industry/healthcare-it
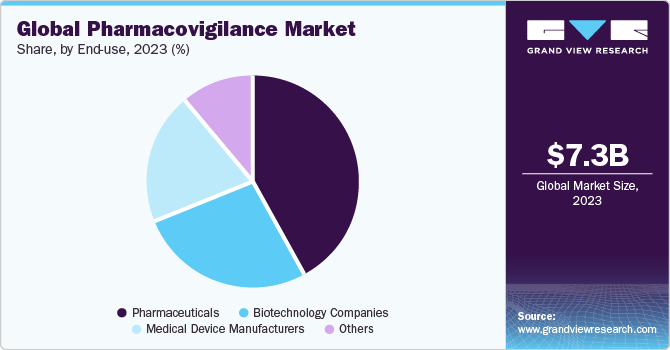
Global pharmacovigilance market revenue, by region, 2016 (%)
Based on type of methods, the market for pharmacovigilance can be classified into intensified spontaneous reporting, spontaneous reporting, targeted spontaneous reporting, cohort event monitoring, and Electronic Health Record (EHR) mining. In 2016, spontaneous reporting held the highest market share attributed to increasing use in detection of rare ADRs. Rising demand for surveillance reports prepared through this method among regulatory authorities and pharmaceutical companies can drive growth of segment.
Regional segmentation includes North America, Europe, Latin America, Asia Pacific, and Middle East and Africa. In 2016, North America held the largest market share. Presence of major medical device manufacturers and pharmaceutical players can be attributed to regional market growth. Increasing number of deaths owing to high consumption of drugs can further fuel regional market. Rising investments by leading market players to develop new drugs can also impel growth in near future. Increasing number of clinical trials and demand for post-marketing surveillance attributed to huge production of drugs can drive market expansion in the forthcoming years.
Asia Pacific is projected to expand at a significant CAGR of 15.1% owing to the presence of a large number of outsourcing organizations. Factors like cost efficiency, better productivity, and resource sharing are anticipated to propel demand for pharmacovigilance in the region. In addition, growing investments and awareness among patients can also spur regional growth in the forthcoming years. Moreover, favorable initiatives by local governments to meet consumer demand can further boost market expansion.
Some of the leading companies operating in the Pharmacovigilance (PV) market are Cognizant, Accenture, Clinquest Group B.V., IBM Corporation, and Laboratory Corporation of America Holdings. Most companies are likely to remodel their manufacturing processes to meet evolving consumer demand. In addition, companies can get involved in outsourcing PV services to reduce operational costs. Moreover, they are expected to adopt business strategies like mergers and acquisitions to maintain their market share.
Grand View Research has segmented the pharmacovigilance market on the basis of the clinical trial phase, service provider, type of methods, end use, and region:
Pharmacovigilance Clinical Trial Phase Outlook (Revenue, USD Million, 2014 – 2025)
-
Pre-clinical
-
Phase I
-
Phase II
-
Phase III
-
Phase IV
Pharmacovigilance Service Provider Outlook (Revenue, USD Million, 2014 – 2025)
-
In-house
-
Contract Outsourcing
Pharmacovigilance Type of Methods Outlook (Revenue, USD Million, 2014 – 2025)
-
Spontaneous Reporting
-
Intensified ADR Reporting
-
Targeted Spontaneous Reporting
-
Cohort Event Monitoring
-
EHR Mining
Pharmacovigilance End Use Outlook (Revenue, USD Million, 2014 – 2025)
-
Hospitals
-
Research Organizations
-
Industrial
Pharmacovigilance Regional Outlook (Revenue, USD Million, 2014 – 2025)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
UK
-
Germany
-
-
Asia Pacific
-
Japan
-
China
-
India
-
-
Latin America
-
Brazil
-
Mexico
-
-
MEA
-
South Africa
-
Explore the BI enabled intuitive market research database, Navigate with Grand View Compass, by Grand View Research, Inc.
About Grand View Research
Grand View Research, Inc. is a U.S. based market research and consulting company, registered in the State of California and headquartered in San Francisco. The company provides syndicated research reports, customized research reports, and consulting services. To help clients make informed business decisions, we offer market intelligence studies ensuring relevant and fact-based research across a range of industries, from technology to chemicals, materials and healthcare.
For more information: www.grandviewresearch.com/
Media Contact
Company Name: Grand View Research, Inc.
Contact Person: Sherry James, Corporate Sales Specialist – U.S.A.
Email: Send Email
Phone: 1-415-349-0058, Toll Free: 1-888-202-9519
Address:28 2nd Street, Suite 3036
City: San Francisco
State: California
Country: United States
Website: www.grandviewresearch.com/industry-analysis/pharmacovigilance-industry

