DelveInsight is pleased to announce the release of its in-depth industry report titled “NM-8074 Sales Forecast, and Market Size Analysis – 2034”, offering a comprehensive evaluation of NM-8074 (ruxoprubart), a promising investigational therapy developed by NovelMed Therapeutics for the treatment of rare and severe complement-mediated diseases. The report underscores NM-8074’s strong commercial potential, driven by its differentiated mechanism of action, expanding clinical footprint, and increasing relevance in high-unmet-need hematologic, renal, and autoimmune disorders across the seven major markets (7MM).
Gain exclusive access to detailed 7MM sales forecasts, competitive intelligence, and indication-wise market insights for ruxoprubart through 2034 @ https://www.delveinsight.com/report-store/nm-8074-sale-forecast-and-market-analysis
A First-in-Class Approach Transforming the Complement Therapeutics Landscape
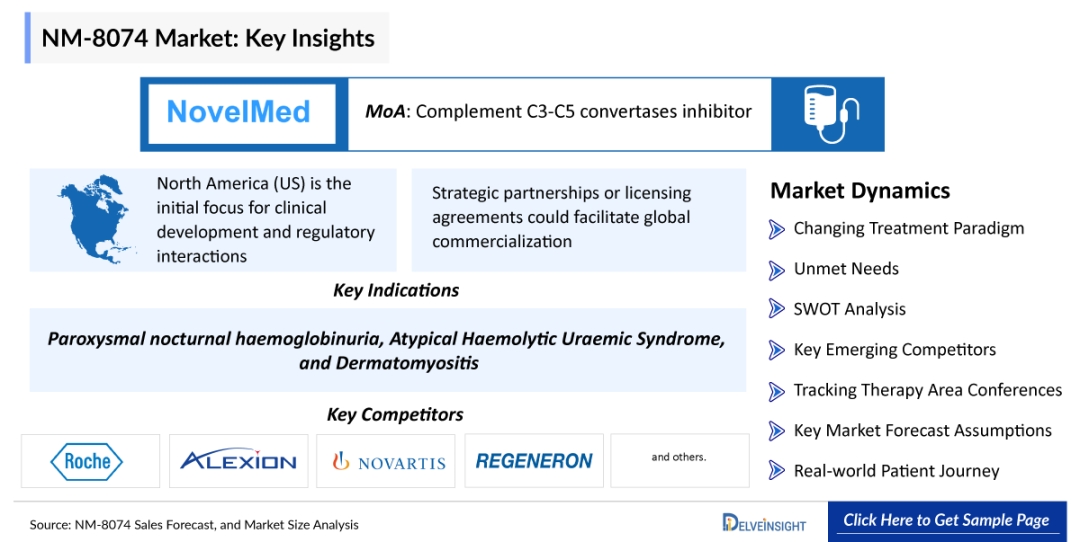
NM-8074, also known as ruxoprubart, is a humanized monoclonal antibody designed to selectively inhibit complement factor Bb, a key serine protease in the alternative complement pathway (AP). Unlike broader complement inhibitors that block C3 or C5 and may compromise essential immune functions, ruxoprubart precisely targets the AP amplification loop while preserving the classical and lectin pathways. This selective inhibition reduces hemolysis, inflammation, and tissue damage while potentially minimizing infection risks associated with broader complement blockade.
This first-in-class anti-Bb mechanism positions NM-8074 as a differentiated and next-generation complement inhibitor, aligning well with emerging market trends favoring precision immunology and targeted biologics.
NM-8074 Sales Forecast Highlights: Sustained Growth Through 2034
According to DelveInsight’s analysis, NM-8074 is projected to achieve steady and scalable sales growth through 2034, supported by:
- Rising new patient starts in treatment-naïve populations
- Expansion into multiple rare and orphan indications
- Strong uptake among hematology, nephrology, and immunology specialists
- Favorable orphan drug incentives and regulatory pathways
The NM-8074 annual sales report provides detailed sales forecasts across the United States, EU4 (Germany, France, Italy, Spain), the United Kingdom, and Japan, covering the period from 2020 to 2034. The US is expected to remain the primary revenue driver in the early years, followed by gradual expansion into Europe and Japan as regulatory engagement progresses.
Speak directly with DelveInsight’s analysts to understand NM-8074’s commercial potential, differentiation strategy, and upcoming regulatory milestones @ NM-8074 Market Forecast and Statistics
Key Factors Driving NM-8074 Market Growth
1. Market Share Gains and New Patient Starts
NM-8074 is strategically positioned to capture market share in the rapidly evolving complement-mediated disease market, which includes rare hematologic and inflammatory disorders with limited treatment options. Its selective AP inhibition may allow it to address both intravascular and extravascular hemolysis, a key limitation of existing therapies.
As clinical development advances, particularly in treatment-naïve patients, new patient starts are expected to increase steadily. Early clinical data in PNH demonstrating hemoglobin stabilization, reduced lactate dehydrogenase (LDH), and transfusion independence further support strong adoption potential post-approval.
2. Expansion Across Multiple High-Value Indications
NM-8074’s development strategy reflects a platform approach, enabling expansion across several complement-driven diseases:
- Paroxysmal Nocturnal Hemoglobinuria (PNH): Currently in Phase II clinical trials, ruxoprubart is being evaluated in complement inhibitor–naïve PNH patients. Interim data have shown encouraging safety and efficacy signals, reinforcing its potential as a future alternative or complement to existing C5 inhibitors.
- Atypical Hemolytic Uremic Syndrome (aHUS): FDA clearance for Phase II initiation in aHUS highlights confidence in targeted AP blockade for thrombotic microangiopathies with high unmet need.
- Complement C3 Glomerulopathy (C3G): Planned Phase Ib development in C3G reflects NM-8074’s growing relevance in rare renal diseases driven by complement dysregulation.
- Dermatomyositis and Other Autoimmune Disorders: Regulatory clearance to initiate Phase II trials in dermatomyositis (DM) marks expansion beyond hematology into chronic inflammatory and autoimmune indications, significantly broadening the drug’s commercial scope.
Collectively, these expansions point to multi-disease, multi-specialty potential, enhancing long-term revenue sustainability.
NM-8074 Regulatory Momentum and Recent Developments
NM-8074 has achieved several key milestones that strengthen its commercial outlook:
- USAN generic name approval as “ruxoprubart”, supporting brand identity and lifecycle progression
- Multiple FDA IND clearances for Phase II trials across PNH, aHUS, C3G, IgA nephropathy, ANCA-associated vasculitis, and dermatomyositis
- Positive 12-week interim Phase II data in PNH announced in May 2025
- FDA clearance in February 2025 to initiate a Phase II efficacy trial in dermatomyositis
These developments significantly expand NM-8074’s future label potential and reinforce confidence among investors, partners, and clinicians.
Explore how NM-8074 is positioned against existing and emerging complement inhibitors and identify opportunities for licensing, partnerships, or portfolio expansion @ NM-8074 (Ruxoprubart) Competitive Landscape
Geographic Expansion and Global Commercialization Strategy for NM-8074
While North America remains the initial focus for clinical development and regulatory engagement, NovelMed is expected to pursue approvals in Europe and Japan, where rare complement-mediated diseases are increasingly recognized and supported by orphan drug frameworks.
Strategic licensing or commercialization partnerships could further accelerate global reach, particularly in Asia-Pacific and other regions with strong rare disease incentives.
Competitive Differentiation in a Crowded Complement Market
The complement inhibitor market has historically been dominated by C5 and C3 inhibitors such as eculizumab. However, ruxoprubart’s precision AP inhibition offers several potential advantages:
- Preservation of key immune functions
- Reduced safety concerns related to infection risk
- Improved tolerability and long-term adherence
- Alignment with biomarker-guided and personalized therapy approaches
Broader industry trends such as increased reliance on real-world evidence (RWE), focus on rare diseases, and biologics innovation further strengthen NM-8074’s positioning.
Leverage in-depth market sizing, pricing analysis, and competitive benchmarking to refine your immunology and rare disease strategy today @ NM-8074 (Ruxoprubart) Sales Forecast
Inside the NM-8074 Market Report
DelveInsight’s NM-8074 market report delivers a 360-degree strategic assessment, including:
- Detailed sales forecasts and market sizing through 2034
- In-depth analysis of mechanism of action, dosing, and administration
- Comprehensive review of clinical trial data and regulatory milestones
- Competitive landscape, emerging therapies, and pipeline analysis
- SWOT analysis, analyst views, and pricing & reimbursement insights
- Evaluation of cost-effectiveness, therapy duration, and regional pricing variations
The report is built using robust primary and secondary research, internal databases, and expert analysis, ensuring high reliability for strategic decision-making.
Why NM-8074 Report Matters
As the complement therapeutics landscape evolves, NM-8074 represents a potential paradigm shift in how rare complement-mediated diseases are treated. DelveInsight’s analysis equips pharmaceutical companies, investors, strategists, and healthcare stakeholders with actionable intelligence to:
- Assess NM-8074’s commercial viability and peak sales potential
- Benchmark competitive positioning against existing and emerging therapies
- Identify licensing, partnership, and portfolio optimization opportunities
- Support data-driven decisions in the immunology and rare disease markets
To access the full “NM-8074 Sales Forecast, and Market Size Analysis – 2034” report and explore how ruxoprubart could reshape the complement-mediated disease market, visit DelveInsight’s Report @ https://www.delveinsight.com/sample-request/nm-8074-sale-forecast-and-market-analysis
About DelveInsight
DelveInsight is a leading provider of life sciences market intelligence, offering syndicated and customized research solutions across pharmaceuticals, biotechnology, and healthcare. Our expertise spans epidemiology, market forecasting, competitive intelligence, pipeline analysis, and strategic consulting.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email: Send Email
Phone: +19193216187
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting