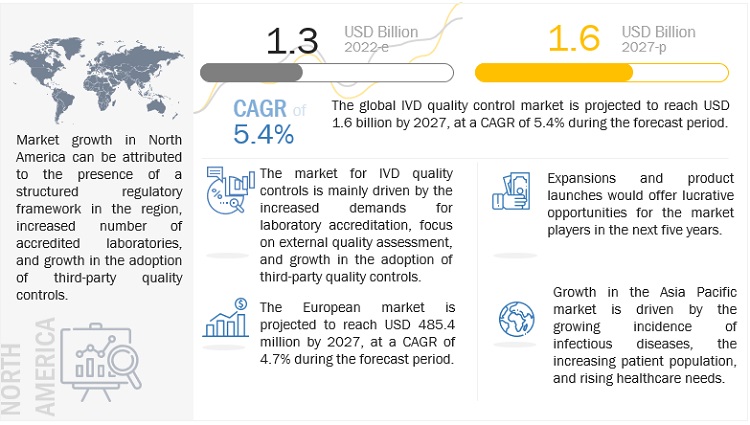
IVD Quality Control Market in terms of revenue was estimated to be worth $1.3 billion in 2022 and is poised to reach $1.6 Billion by 2027, growing at a CAGR of 5.4% from 2022 to 2027 according to a new report by MarketsandMarkets™. The growth of the IVD quality control market is driven by the rising number of accredited clinical laboratories, rising geriatric population and subsequent growth in the prevalence of chronic and infectious diseases and increasing adoption of third-party quality controls. The rising focus on multi-analyte controls and Increasing investments from government bodies and private players in healthcare sectors in emerging economies is also expected to offer significant growth opportunities for the market in the coming years. Lack of regulations for clinical laboratory accreditation in several emerging countries could be the challenges faced by the market in upcoming years.
Request for FREE Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=198032582
Browse in-depth TOC on “IVD Quality Control Market”
254 – Tables
54 – Figures
331 – Pages
The IVD quality control industry is expected to witness a significant growth in near future. This is due to the increasing demand for quality control in the health care sector and the development of new technologies and processes that can help improve accuracy and efficiency.
Key Market Players of IVD Quality Control Industry
Some of the key players in the market include Bio-Rad Laboratories, Inc. (US), Randox Laboratories Ltd. (UK), Thermo Fisher Scientific, Inc. (US), LGC Limited (UK), and Abbott Laboratories (US). Other prominent payers in the market include Roche Diagnostics (Switzerland), Siemens Healthineers (Germany), Danaher Corporation (US), Fortress Diagnostics (UK), SERO AS (US), Sysmex Corporation (Japan), Ortho-Clinical Diagnostics (US), Helena Laboratories Corporation (US), Quidel Corporation (US), Sun Diagnostics, LLC (US), Seegene Inc. (South Korea), ZeptoMetrix Corporation (US), Qnostics (UK), Bio-Techne Corporation (US), Microbiologics (US), Microbix Biosystems (Canada), Streck, Inc. (US), Alpha-Tec Systems (US), Maine Molecular Quality Controls, Inc. (US), and Grifols, S.A. (Spain).
North America to dominate the global IVD quality control market
North America dominated the global IVD quality control market. Approvals of quality control products from the FDA and the College of American Pathologists (CAP) and the presence of leading companies in the US are driving the IVD quality control market in North America. US dominated the North America IVD quality control market. In the coming years, the increasing number of IVD tests; the growing need to ensure the accuracy, reliability, and reproducibility of test results; and the use of third-party quality controls among clinical laboratories in this region will play a key role in the market growth.
Get 10% Free Customization on this Report: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=198032582
IVD Quality Control Market Dynamics:
Drivers:
- Increasing number of accredited clinical laboratories
- Growing adoption of third-party quality controls
- Rising demand for external quality assessment support
- Rising geriatric population and subsequent growth in the prevalence of chronic and infectious diseases
- Increasing adoption of POC instruments in developed regions
Restraints:
- Additional costs and budget constraints in hospitals and laboratories
- Unfavorable reimbursement scenario for IVD tests
Opportunities:
- Rising demand for multi-analyte controls
Challenges:
- Stringent product approval process
- Lack of regulations for clinical laboratory accreditation in several emerging countries
The study categorizes the global IVD quality control market to forecast revenue and analyze trends in each of the following submarkets:
By Product and Service
- Quality control products
- Serum/ Plasma based controls
- Whole blood based controls
- Urine based controls
- Other controls
- Data Management Solutions
- Quality Assurance Services
By Technology
- Immunochemistry
- Clinical Chemistry
- Molecular Diagnostics
- Microbiology
- Hematology
- Coagulation/ Hemostasis
- Other Technologies
By Manufacturer Type
- Third-party controls
- Independent controls
- Instrument specific controls
- Original Equipment Manufacturer controls
By End User
- Hospitals
- Clinical Laboratories
- Academic and Research Institutes
- Other End users
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Make an Enquire to buy This Report @ https://www.marketsandmarkets.com/Enquiry_Before_BuyingNew.asp?id=198032582
About MarketsandMarkets™:
MarketsandMarkets™ has been recognized as one of America’s best management consulting firms by Forbes, as per their recent report.
MarketsandMarkets™ is a blue ocean alternative in growth consulting and program management, leveraging a man-machine offering to drive supernormal growth for progressive organizations in the B2B space. We have the widest lens on emerging technologies, making us proficient in co-creating supernormal growth for clients.
Earlier this year, we made a formal transformation into one of America’s best management consulting firms as per a survey conducted by Forbes.
The B2B economy is witnessing the emergence of $25 trillion of new revenue streams that are substituting existing revenue streams in this decade alone. We work with clients on growth programs, helping them monetize this $25 trillion opportunity through our service lines – TAM Expansion, Go-to-Market (GTM) Strategy to Execution, Market Share Gain, Account Enablement, and Thought Leadership Marketing.
Built on the ‘GIVE Growth’ principle, we work with several Forbes Global 2000 B2B companies – helping them stay relevant in a disruptive ecosystem. Our insights and strategies are molded by our industry experts, cutting-edge AI-powered Market Intelligence Cloud, and years of research. The KnowledgeStore™ (our Market Intelligence Cloud) integrates our research, facilitates an analysis of interconnections through a set of applications, helping clients look at the entire ecosystem and understand the revenue shifts happening in their industry.
Media Contact
Company Name: MarketsandMarkets™ Research Private Ltd.
Contact Person: Mr. Aashish Mehra
Email: Send Email
Phone: 18886006441
Address:630 Dundee Road Suite 430
City: Northbrook
State: IL 60062
Country: United States
Website: https://www.marketsandmarkets.com/Market-Reports/in-vitro-diagnostics-quality-controls-market-198032582.html