DelveInsight’s ‘Head and Neck Cancer Pipeline Insight 2022‘ report provides comprehensive global coverage of available, marketed, and pipeline head and neck cancer therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the head and neck cancer pipeline domain.
Key Takeaways from the Head and Neck Cancer Pipeline Report
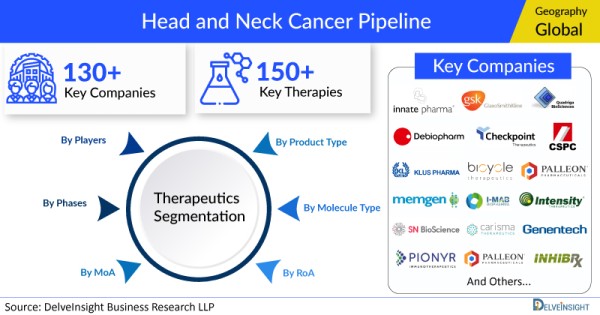
- DelveInsight’s head and neck cancer pipeline report depicts a robust space with 130+ active players working to develop 150+ pipeline therapies for head and neck cancer treatment.
- Key head and neck cancer companies such as Innate Pharma, GlaxoSmithKline, Debiopharm, Checkpoint Therapeutics, Quadriga BioSciences, Palleon Pharmaceuticals, Klus Pharma, Bicycle Therapeutics, CSPC ZhongQi Pharmaceutical Technology, Memgen, I-Mab Biopharma, Intensity Therapeutics, Wellmaker Bio, SN BioScience, Carisma Therapeutics, Genentech, Pionyr Immunotherapeutics, Palleon Pharmaceuticals, SN BioScience, Bicycle Therapeutics, Inhibrx, Celldex Therapeutics, Carisma Therapeutics Inc, BioAtla, Jacobio Pharmaceuticals, Compugen, GeoVax, CytomX Therapeutics, PNP Therapeutics, Galectin Therapeutics, Cyteir Therapeutics, Merck KGaA, Boehringer Ingelheim, Transgene, iTeos Therapeutics SA, IntraGel Therapeutics, Incyte Corporation, Cue Biopharma, Rubius Therapeutics, Alkermes, Corregene Biotechnology, NanoCarrier, Kura Oncology, Betta Pharmaceuticals, Arcus Biosciences, Taiho Pharmaceutical, Astex Pharmaceuticals, Bayer HealthCare, Roche, Vaccinex, Pfizer, BioNTech, Purple Biotech, Novartis Oncology, Macrogenics, Aveta Biomics, Cellectar Biosciences, Nanobiotix, Nektar Therapeutics, Istari Oncology, Nanobiotix, Aspyrian Therapeutics, and others are evaluating new head and neck cancer drugs to improve the treatment landscape.
- Promising head and neck cancer pipeline therapies such as Niraparib, Xevinapant, , CK-301, QBS10072S, A166, E-602, BT5528, RAPA-201, Mitoxantrone Hydrochloride, MEM-288, TJ004309, SNB-101, INT230-6, CT-0508, WM-S1-030, CDX-1140, PY159, MCLA-129, INBRX-106, Autogene cevumeran, JAB-3068, CAB-ROR2-ADC, CX-2029, Ad/PNP, COM701, Eftilagimod alpha, CYT-0851, GR-MD-02, TG4050, BI 765063, TumoCure, EOS-448, Epacadostat, RTX-321, CUE-101, ALKS 4230, CRTE7A2-01, NC-6004, Tipifarnib, MCLA-129, Zimberelimab, HLX10 Plus HLX07, Tolinapant, BAY1905254, OSI-774, VX15/2503, Palbociclib, Olaparib, NT219, BNT113, Buparlisib, Retifanlimab, Avelumab, APG-157, CLR 131, NBTXR3, Evorpacept, Dostarlimab, NKTR-214, IK-175, Lerapolturev, NBTXR3, Gedeptin, RM-1995, Atezolizumab and others are under different phases of head and neck cancer clinical trials.
- In September 2022, NANOBIOTIX announced randomization of the first patient in Asia in NANORAY-312, a global Phase III registrational trial evaluating NBTXR3 for the treatment of elderly patients with locally advanced head and neck squamous cell carcinoma who are ineligible for platinum-based chemotherapy.
- In August 2022, Genexine announced the dosing of the first patient in Phase II clinical trial using triple combination therapy in patients with recurrent/metastatic HNSCC. The combination therapy consists of two of Genexine’s proprietary drugs, GX-188E (a first-in-class therapeutic DNA vaccine), GX-I7 (a first-in-class long-acting interleukin 7), and Opdivo (nivolumab), a marketed PD-1 immune checkpoint inhibitor.
- In May 2022, Calliditas announced that it had initiated Phase II head and neck cancer therapy trial. The trial will analyze the impact of setanaxib plus pembrolizumab in patients with CAF-density tumors. Calliditas Therapeutics has randomized the first subject in the proof-of-concept Phase II clinical trial of setanaxib (GKT831) in individuals with squamous cell carcinoma of the head and neck (SCCHN).
- In May 2022, Exelixis announced results from a phase II, the investigator-sponsored trial of cabozantinib (CABOMETYX®) in combination with pembrolizumab in patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). The trial met its primary endpoint of objective response rate per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 at 54%. The overall clinical benefit rate was 91%. At a median follow-up of 10.6 months, the one-year progression-free survival rate was 54.0% (95% confidence interval [CI]: 31.5-72.0%), and median progression-free survival was 14.6 months. The one-year overall survival (OS) rate was 68.4% (95% CI: 45.1-83.5%; median OS: 22.3 months).
- In January 2022, The FDA accepted an investigational new drug application for the photoimmunotherapy treatment, RM-1995, for patients with advanced cutaneous squamous cell carcinoma or head and neck squamous cell carcinoma.
- In February 2021, The Food and Drug Administration (FDA) granted Breakthrough Therapy designation to tipifarnib for the treatment of patients with recurrent or metastatic HRAS mutant head and neck squamous cell carcinoma with variant allele frequency greater than or equal to 20% after disease progression on platinum-based chemotherapy.
Request a sample and discover the recent advances in head and neck cancer treatment drugs @ Head and Neck Cancer Pipeline Report
The head and neck cancer pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage head and neck cancer drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the head and neck cancer clinical trial landscape.
Head and Neck Cancer Overview
Head and neck cancer is a collective term for several different types of cancer. Cancers of the head and neck are classified according to where they begin. The mouth (oral cavity), throat (pharynx), voice box (larynx), sinuses and nose cavity, and salivary glands are all included. Squamous cell carcinoma of the head and neck is the most common type of head and neck cancer (HNSCCA). The majority of HNSCCA begins in the layer of flat cells (the epithelium) that lines the upper aerodigestive tract structures such as the mouth, throat, and voice box.
Head and neck cancer symptoms include a lump in the neck or a sore in the mouth or throat that does not heal, a persistent sore throat, difficulty swallowing, and a change or hoarseness in voice; however, these head and neck cancer symptoms can also be caused by other, less severe illnesses.
For head and neck diagnosis, physical examination/blood and urine tests, endoscopy, biopsy, biomarker testing of the tumor, X-ray/barium swallow, panoramic radiograph, ultrasound, magnetic resonance imaging, bone scan, and other tests are used.
Learn more about the emerging head and neck cancer pipeline therapies @ Head and Neck Cancer Clinical Trials
Head and Neck Cancer Therapeutics Assessment
The head and neck cancer pipeline report proffers an integral view of the head and neck cancer emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Head and Neck Cancer Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Inhalation, Intranasal, Intravenous, Oral, Parenteral, Subcutaneous
- Therapeutics Assessment By Molecule Type: Antibody, Antisense oligonucleotides, Immunotherapy, Monoclonal antibody, Peptides, Protein, Recombinant protein, Small molecule, Stem cell, Vaccine
- Therapeutics Assessment By Mechanism of Action: Antibody-dependent cell cytotoxicity, NK cell lectin-like receptor subfamily C antagonists, Poly(ADP-ribose) polymerase 1 inhibitors, Poly(ADP-ribose) polymerase 2 inhibitors, Inhibitor of apoptosis protein inhibitors, T lymphocyte stimulants, Aryl hydrocarbon receptor antagonists, DNA topoisomerase I inhibitors; CD40 ligand expression stimulants; Cell death stimulants; Immunologic cytotoxicity, Galectin 3 inhibitors, CD40 antigen stimulants
- Key Head and Neck Cancer Companies: Innate Pharma, GlaxoSmithKline, Debiopharm, Checkpoint Therapeutics, Quadriga BioSciences, Palleon Pharmaceuticals, Klus Pharma, Bicycle Therapeutics, CSPC ZhongQi Pharmaceutical Technology, Memgen, I-Mab Biopharma, Intensity Therapeutics, Wellmaker Bio, SN BioScience, Carisma Therapeutics, Genentech, Pionyr Immunotherapeutics, Palleon Pharmaceuticals, SN BioScience, Bicycle Therapeutics, Inhibrx, Celldex Therapeutics, Carisma Therapeutics Inc, BioAtla, Jacobio Pharmaceuticals, Compugen, GeoVax, CytomX Therapeutics, PNP Therapeutics, Galectin Therapeutics, Cyteir Therapeutics, Merck KGaA, Boehringer Ingelheim, Transgene, iTeos Therapeutics SA, IntraGel Therapeutics, Incyte Corporation, Cue Biopharma, Rubius Therapeutics, Alkermes, Corregene Biotechnology, NanoCarrier, Kura Oncology, Betta Pharmaceuticals, Arcus Biosciences, Taiho Pharmaceutical, Astex Pharmaceuticals, Bayer HealthCare, Roche, Vaccinex, Pfizer, BioNTech, Purple Biotech, Novartis Oncology, Macrogenics, Aveta Biomics, Cellectar Biosciences, Nanobiotix, Nektar Therapeutics, Istari Oncology, Nanobiotix, Aspyrian Therapeutics and others.
- Key Head and Neck Cancer Pipeline Therapies: Niraparib, Xevinapant, , CK-301, QBS10072S, A166, E-602, BT5528, RAPA-201, Mitoxantrone Hydrochloride, MEM-288, TJ004309, SNB-101, INT230-6, CT-0508, WM-S1-030, CDX-1140, PY159, MCLA-129, INBRX-106, Autogene cevumeran, JAB-3068, CAB-ROR2-ADC, CX-2029, Ad/PNP, COM701, Eftilagimod alpha, CYT-0851, GR-MD-02, TG4050, BI 765063, TumoCure, EOS-448, Epacadostat, RTX-321, CUE-101, ALKS 4230, CRTE7A2-01, NC-6004, Tipifarnib, MCLA-129, Zimberelimab, HLX10 Plus HLX07, Tolinapant, BAY1905254, OSI-774, VX15/2503, Palbociclib, Olaparib, NT219, BNT113, Buparlisib, Retifanlimab, Avelumab, APG-157, CLR 131, NBTXR3, Evorpacept, Dostarlimab, NKTR-214, IK-175, Lerapolturev, NBTXR3, Gedeptin, RM-1995, Atezolizuma, and others.
Dive deep into rich insights for new drugs for head and neck cancer treatment, visit @ Head and Neck Cancer Drugs
Table of Contents
1. Head and Neck Cancer Pipeline Report Introduction
2. Head and Neck Cancer Pipeline Report Executive Summary
3. Head and Neck Cancer Pipeline: Overview
4. Analytical Perspective In-depth Commercial Assessment
5. Head and Neck Cancer Clinical Trial Therapeutics
6. Head and Neck Cancer Pipeline: Late Stage Products (Phase III)
6.1 Atezolizumab: Genentech
7. Head and Neck Cancer Pipeline: Late Stage Products (Phase II)
7.1 A166: Klus Pharma
8. Head and Neck Cancer Pipeline: Mid Stage Products (Phase I/II)
8.1 BT5528: Bicycle Therapeutics
9. Head and Neck Cancer Pipeline: Early Stage Products (Phase I)
9.1 CDX-1140: Celldex Therapeutics
10. Head and Neck Cancer Pipeline Therapeutics Assessment
11. Inactive Products in the Head and Neck Cancer Pipeline
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Key Companies
14. Key Products in the Head and Neck Cancer Pipeline
15. Unmet Needs
16. Market Drivers and Barriers
17. Future Perspectives and Conclusion
18. Analyst Views
19. Appendix
For further information on the head and neck cancer pipeline therapeutics, reach out @ Head and Neck Cancer Treatment Drugs
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email: Send Email
Phone: +19193216187
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting

