InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global Endovascular Abdominal Aortic Aneurysm Repair Devices Market – (By Product Type (Synthetic Fabric Graft, Stent-graft), By Age Group (Adult, Geriatric, Pediatric), By Material (Polymer, Metal), By Aortic Length (Above 50 mm, Above 100 mm, By End-User (Hospital, Ambulatory Surgical Centers (ASCs), Research Institutes, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031.”
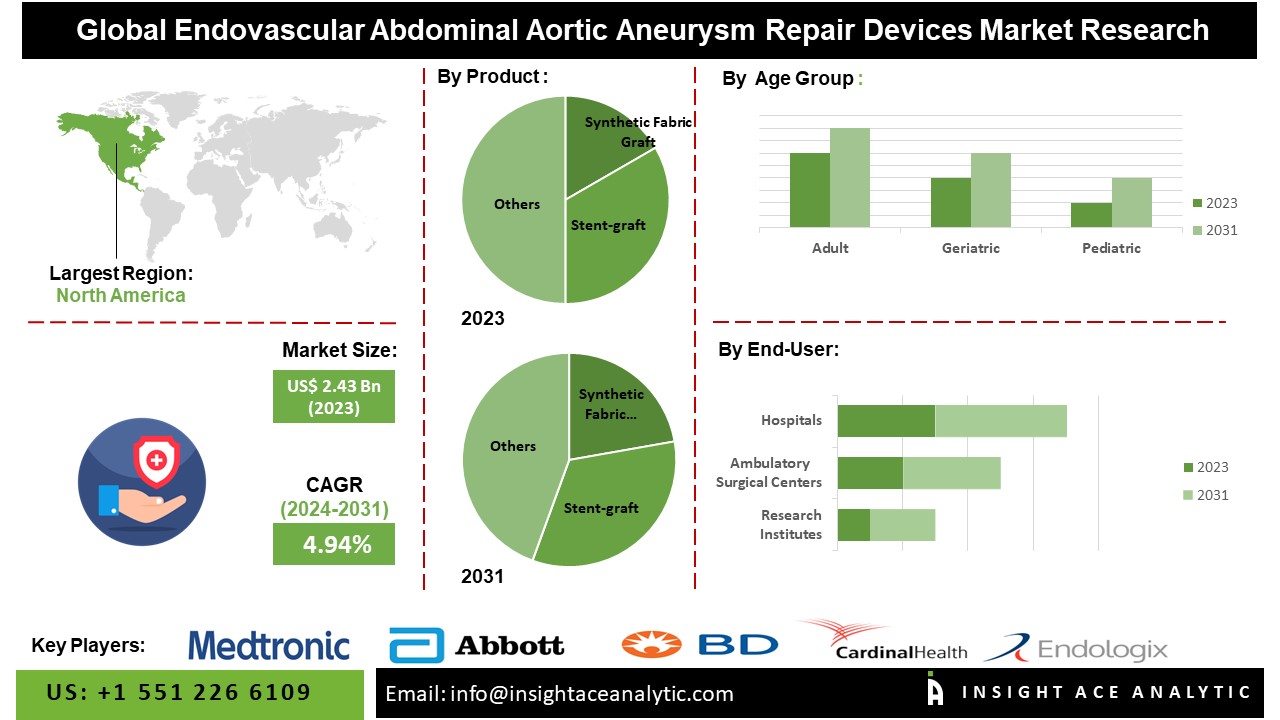
According to the latest research by InsightAce Analytic, the Global Endovascular Abdominal Aortic Aneurysm Repair Devices Market is valued at US$ 2.43 Bn in 2023, and it is expected to reach US$ 3.53 Bn by 2031, with a CAGR of 4.9% during the forecast period of 2024-2031.
Endovascular devices for repairing abdominal aortic aneurysms (AAA) have significantly enhanced the treatment of this condition. They provide a safer and less intrusive option compared to standard open surgery for many patients. The main constituent of the stent graft is a cylindrical structure made of fabric that is reinforced by a metal framework known as a stent. Its purpose is to be inserted into the aneurysm in order to establish an alternative route for the circulation of blood, so successfully separating the aneurysm from blood pressure and averting its rupture. Growing rates of abdominal aortic aneurysms and improvements in endovascular procedures are driving significant growth in this market. Given its benefits over open surgery, endovascular aneurysm repair (EVAR) is recommended as a therapy option for aortic aneurysms. Shorter hospital stays, faster recovery times, better patient reactions, and no surgery are some of these benefits. As a result, the EVAR technique is a conventional intervention for abdominal aortic aneurysms. By doing this, the blood flow pressure inside the aneurysmal sac is kept from rupturing. The need for less intrusive treatments is fueling the need for novel gadgets. Innovations in technology, such as catheters and stent-grafts, improve patient safety and therapeutic results.
Buy Now This Report: https://www.insightaceanalytic.com/buy-report/2400
List of Prominent Players in the Endovascular Abdominal Aortic Aneurysm Repair Devices Market:
- Terumo Medical Corporation
- Medtronic Plc
- Becton Dickinson and Company
- Koninklijke Philips N.V.
- Lombard Medical Inc.
- Endologix Inc.
- Abbott Laboratories
- Cardinal Health Inc.
- W. L. Gore & Associates Inc
- Boston Scientific Corporation
- Cook Group Incorporated
- Other Prominent Players
Market Dynamics:
Drivers-
The increase in the prevalence of abdominal aortic aneurysm (AAA) and advancement in endovascular technology are the key factors driving the global market share of endovascular abdominal aortic aneurysm repair devices in the next few years. The increase in the geriatric population, growth in the adoption of unhealthy lifestyles, and increase in obesity rates are also contributing to market growth and increasing awareness about the condition and availability of advanced treatment options, further augmenting market statistics. The rise in the adoption of endovascular AAA repair devices in emerging economies creates lucrative business opportunities for market players in endovascular abdominal aortic aneurysm repair devices. Leading companies focus on developing new products using advanced technologies to expand their global footprint.
Challenges:
The Endo-vascular Abdominal Aortic Aneurysm Repair Device Market faces several constraints, including stringent regulatory requirements for approval, high initial costs of devices and procedures limiting accessibility, and specialized training among healthcare professionals for proper deployment and maintenance. Additionally, technological advancements and innovation in traditional surgical techniques pose competitive challenges. Economic constraints, such as reimbursement policies and healthcare budget limitations, further impact market growth. Moreover, concerns regarding long-term device durability and potential complications necessitate continuous research and development efforts to address safety and efficacy issues, adding to market restraints.
Regional Trends:
The North American Endovascular Abdominal Aortic Aneurysm Repair Device Market is projected to hold a prominent market share in revenue and is envisioned to gain a high CAGR soon. Growing population, rapid urbanization, and increasing industrialization, coupled with increasing R&D activities by prominent players, are factors expected to increase the market growth in the region. Besides, Europe had a strong market share due to the developed economy and increasing product demand. This is because of the new strategies by the major players in the country and the increase in EVAR procedures. Further, the participation of essential market companies and increasing collaboration among major players provide this market’s growth opportunity.
Curious About This Latest Version Of The Report? Enquiry Before Buying: https://www.insightaceanalytic.com/enquiry-before-buying/2400
Recent Developments
- In Jan 2023, Medtronic has initiated the first phase of a worldwide randomized trial to assess the long-term effectiveness of endovascular aneurysm repair. The ADVANCE Trial was a worldwide, post-market, forward-looking, interventional, multicenter, randomized research that recruited at least 550 participants at a maximum of 50 locations around the world.
- In Oct 2021, Terumo Aortic has received regulatory approval and has officially launched the Treo abdominal aortic stent transplant system in Japan. The Treo device has been authorized by the Japanese Pharmaceuticals & Medical Devices Agency for the treatment of individuals suffering from abdominal aortic aneurysms.
Segmentation of Endovascular Abdominal Aortic Aneurysm Repair Devices Market-
By Product Type-
- Synthetic Fabric Graft
- Stent-graft
By Age Group-
- Adult
- Geriatric
- Pediatric
By Material
- Polymer
- Metal
By Aortic Length
- Above 50 mm
- Above 100 mm
By End-User
- Hospital
- Ambulatory Surgical Centers (ASCs)
- Research Institutes
- Others
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- South East Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/2400
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/