The global endoluminal suturing devices market is anticipated to be worth USD 103.8 million by the year 2027, advancing at a healthy CAGR of 12.0% during the forecast period, according to a recent Grand View Research report. Endoluminal surgery is a minimally invasive procedure that is performed in a hollow organ through surgical techniques like suturing and stapling, which helps in weight loss for the obese population. It has the potential to be a much safer and cost-effective ambulatory weight loss procedure than the present conventional laparoscopy ones. In order to promote volume reduction as well and add possible elements of malabsorption, manufacturers are currently putting in efforts to develop endoscopic suturing and stapling devices, including the concepts of endoluminal gastroplasties, restrictive valves, and combined approaches to restriction and malabsorption.
The growing incidences of obesity across the globe, coupled with the increasing preference for minimally invasive surgeries, are expected to boost the endoluminal suturing devices market in the coming years, which is still at a nascent stage. The issue of obesity is particularly very pronounced in the United States, where it is the second most-preventable cause of death. According to the WHO, the global obesity prevalence almost tripled in the period between 1975 and 2016, with 650 million adults suffering from the disorder. These people are at a very high risk of suffering from fatal cardiovascular diseases, and may face an early demise, which has been proven in many scientific studies.
Get Details For “Sample Report, TOC, Segmentation & methodology of Endoluminal Suturing Devices Market” Click Link Below: https://www.grandviewresearch.com/industry-analysis/endoluminal-suturing-devices-market
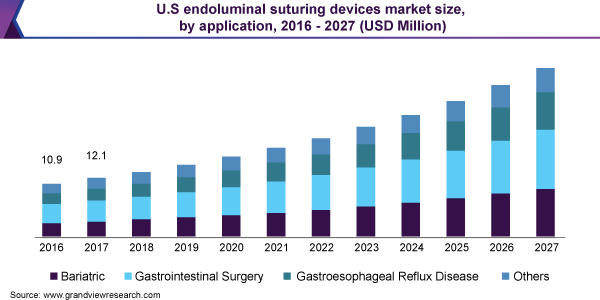
A major factor that is driving the endoluminal suturing devices market is the technological advancements in the endoluminal devices, which has enabled doctors and clinicians to explore various weight loss procedures, along with gastrointestinal surgeries. These surgeries have been in high demand among the overweight and obese demographics, as they help in preventing the development of other major disorders. Another factor positively affecting market growth is the integration of robot-assisted surgery modalities in gastroenterology, which is expected to make endoluminal procedures highly precise and efficient. The technique of robotic suturing gives surgeons a degree of freedom of robotic arm while also providing 3D vision. Their presence has allowed doctors and surgeons to explore the various applications of endoluminal surgeries.
The endoluminal suturing devices market has been broadly segmented into application, end-use and region. Based on application, the market mainly includes bariatric surgery, gastrointestinal surgery and gastroesophageal reflux disease surgery, among others. The increase in number of gastrointestinal diseases in recent years saw the segment dominate the market in 2019, with these diseases affecting a number of major organs such as liver, pancreas, biliary system and alimentary canal, among others. Bariatric surgeries are also expected to show healthy growth in the coming years, owing to the rising cases of obesity globally. According to the ASMBS, the year 2017 saw around 228,000 bariatric procedures being performed in the U.S., with the number increasing to 252,000 in 2018.
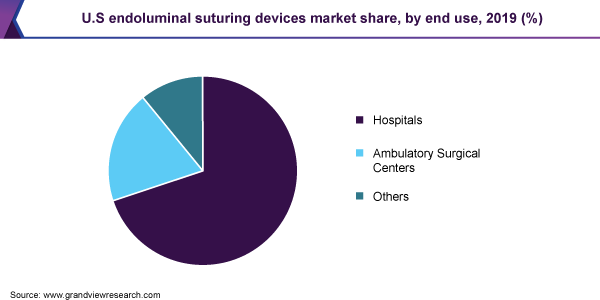
Based on end-use, the market has been classified into hospitals and ambulatory surgical centers (ASCs), among others. The hospitals segment led the market in 2019, contributing to nearly 70% of the overall revenue. Some of the major factors aiding the development of this end-use segment include the rising number of hospital admissions for bariatric surgeries, along with the improving healthcare infrastructure and favorable government initiatives. Meanwhile, ASCs are expected to show the fastest growth in the coming years, on account of the lower costs incurred as opposed to hospital stay, as well as technological advances witnessed in the space of endoluminal surgeries, thus enabling higher number of outpatient procedures.
Would you Like to Take a Look On “Sample Report” of Endoluminal Suturing Devices Market Click the Link Below:
https://www.grandviewresearch.com/industry-analysis/endoluminal-suturing-devices-market/request/rs1
Have Any Query? Ask Our Experts @ https://www.grandviewresearch.com/inquiry/450384/ibb
Trends and Developments:
-
Apollo Endosurgery announced the 510k submission of the X-Tack™ Endoscopic HeliX Tacking System to the U.S. FDA in July 2020, seeking clearance for a through-the-scope, suture-based device specifically designed to close defects in the lower GI tract, with additional applications in the upper GI tract. With the US witnessing around 20 million endoscopic procedures in the lower GI tract each year, the clearance for X-Tack would allow physicians to effectively address closure challenges of commonly encountered large or irregularly shaped defects.
-
Apollo Endosurgery received 510(k) clearance from the U.S. Food and Drug Administration for its Polypropylene Suture-Anchor Assembly in September 2019, specifically for use with the OverStitch™ Endoscopic Suturing Systems. The OverStitch and OverStitch Sx endoscopic suturing systems offer a wide range of less invasive solutions for physicians treating defects in the upper and lower gastrointestinal tract of patients, along with performing a number of advanced bariatric procedures.
-
Asia Pacific and North America are anticipated to account for majority of the market revenue in the coming years. The former region is expected to become an attractive market for endoluminal suturing devices, on account of the improving healthcare infrastructure, along with the growing proportion of the target population. Meanwhile, North America has a huge population base suffering from obesity and weight-related disorders, which has increased the importance of bariatric surgeries in the region. Moreover, the favorable reimbursement policies along with constant developments in the field by government and private organizations is expected to drive this regional market further.
Request Customization Report of Endoluminal Suturing Devices Market @ https://www.grandviewresearch.com/request-for-customization/450384/rfc1
About Grand View Research
Grand View Research provides syndicated as well as customized research reports and consulting services on 46 industries across 126 major countries worldwide. This U.S.-based market research and consulting company is registered in California and headquartered in San Francisco. Comprising over 4126 analysts and consultants, the company adds 121200+ market research reports to its extensive database each year. Supported by an interactive market intelligence platform, the team at Grand View Research guides Fortune 600 companies and prominent academic institutes in comprehending the global and regional business environment and carefully identifying future opportunities
-
urgery announced the 510k submission of the X-Tack™ Endoscopic HeliX Tacking System to the U.S. FDA in July 2020, seeking clearance for a through-the-scope, suture-based device specifically designed to close defects in the lower GI tract, with additional applications in the upper GI tract. With the US witnessing around 20 million endoscopic procedures in the lower GI tract each year, the clearance for X-Tack would allow physicians to effectively address closure challenges of commonly encountered large or irregularly shaped defects.
-
Apollo Endosurgery received 510(k) clearance from the U.S. Food and Drug Administration for its Polypropylene Suture-Anchor Assembly in September 2019, specifically for use with the OverStitch™ Endoscopic Suturing Systems. The OverStitch and OverStitch Sx endoscopic suturing systems offer a wide range of less invasive solutions for physicians treating defects in the upper and lower gastrointestinal tract of patients, along with performing a number of advanced bariatric procedures.
-
Asia Pacific and North America are anticipated to account for majority of the market revenue in the coming years. The former region is expected to become an attractive market for endoluminal suturing devices, on account of the improving healthcare infrastructure, along with the growing proportion of the target population. Meanwhile, North America has a huge population base suffering from obesity and weight-related disorders, which has increased the importance of bariatric surgeries in the region. Moreover, the favorable reimbursement policies along with constant developments in the field by government and private organizations is expected to drive this regional market further.
Media Contact
Company Name: Grand View Research, Inc.
Contact Person: Sherry James, Corporate Sales Specialist – U.S.A.
Email: Send Email
Phone: 1-415-349-0058, Toll Free: 1-888-202-9519
Address:201, Spear Street, 1100
City: San Francisco
State: California
Country: United States
Website: https://www.grandviewresearch.com/industry-analysis/endoluminal-suturing-devices-market

