The Diabetic Macular Edema market report provides current treatment practices, emerging drugs, DME market share of the individual therapies, current and forecasted DME market size from 2018 to 2030 segmented by seven major markets. The Report also covers current DME treatment practice/algorithm, market drivers, market barriers, and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the market.
Diabetic Macular Edema Overview
DME is triggered by diabetic retinopathy (DR), a well-known complication of diabetes. DR is the most prevalent diabetic eye disease and the leading cause of irreversible blindness in people in their working years in the developed and other developing countries. DR is caused by long-term damage to the retina’s small blood vessels. The leakage of fluid into the retina may lead to swelling of the surrounding tissue, including the macula. If left untreated, fluid can leak into the macula’s center, called the fovea, the part of the eye where sharp, straight-ahead vision occurs. The fluid makes the macula swell, blurring vision. This condition is called DME. The definition of clinically significant macular edema (CSME) is the most significant outcome of the Early Treatment Diabetic Retinopathy Study (ETDRS); it established a method for classifying and diagnosing DME and determining when treatment is required.
Request free sample copy- https://www.delveinsight.com/sample-request/diabetic-macular-edema-dme-market
Regions Covered in the report
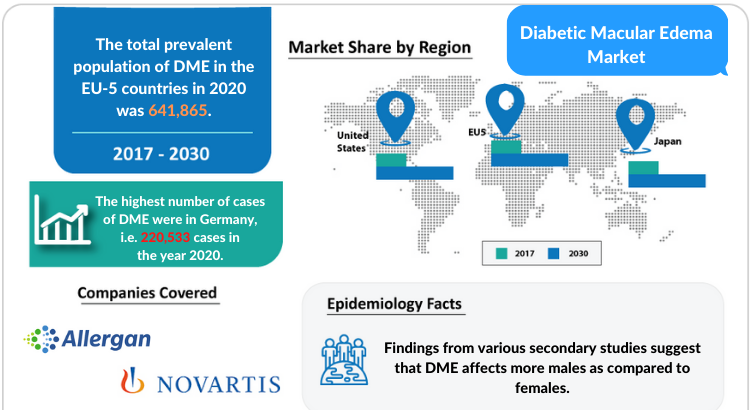
- The United States
- EU5 (Germany, France, Italy, Spain, and the United Kingdom)
- Japan
List of companies involved in the report
- Adverum Biotechnologies
- Graybug Vision
- Roche
- Novartis
- KalVista Pharmaceuticals
- Ocuphire Pharma
- Oxurion
- YD Life Science
- Novartis
- Allergan (AbbVie)/Molecular Partners
- Allergo Opthalmics/ Bausch Health
- Kodiak Sciences
- Clearside Biomedical
- And many others
Diabetic Macular Edema Diagnosis
For the diagnosis of CSME, one of the following characteristics must be present on clinical examination: Any retinal thickening within 500 μm of the center of the macula. Hard exudates within 500 μm of the center of the macula with adjacent retinal thickening. Retinal thickening at least 1 disc area in size, any part of which is within 1 disc diameter of the macula’s center. DME diagnosis is clinically performed by fundoscopy; when the center of the macula (fovea centralis) is thickened or swollen, this is referred to as a clinically significant central-involved macular edema (CI-CSME). It is referred to as a clinically significant non-central-involved macular edema (NCI-CSME) when it is unaffected. Apart from this, tests that help in the diagnosis of DME are visual acuity, refractive error examination, fluorescein angiography (FA), optical coherence tomography (OCT) scan, pupil examination, slit-lamp examination, and retinal examination. However, if the diagnosis is delayed or the condition is left untreated, complications can arise and may even permanently impair the vision.
Diabetic Macular Edema Treatment
Treatment for DME mainly involves the usage of anti-VEGF Drugs, corticosteroids, and Laser photocoagulation. The current standard of care for DME is intravitreal injection. Nonsteroidal anti-inflammatory drugs (NSAIDs), in the form of eye drops, are sometimes used either before or after cataract surgery to prevent the development of macular edema. Currently, intravitreal anti-VEGF agents are the preferred first-line treatment for DME. The three most used anti-VEGF drugs are Avastin (off-label), Eylea, and Lucentis. Corticosteroids are mostly a second-line treatment option. These anti-inflammatory drugs are usually administered via eye drops, pills, or injections of sustained-release corticosteroids into or around the eye. The US FDA approved sustained-release corticosteroid implants for more serious or longer-lasting conditions are Ozurdex, and Iluvien.
Diabetic Macular Edema Drug Chapters
Drug chapter segment of the DME report encloses the detailed analysis of DME developmental stage pipeline drugs. It also helps to understand the DME clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Diabetic Macular Edema Marketed Therapies
- Lucentis (Ranibizumab): Genentech/Novartis
- Eylea (Aflibercept): Bayer/Regeneron Pharmaceuticals/Santen
- And many others
Diabetic Macular Edema Emerging Drugs
- Faricimab (RG 7716): Roche
- Beovu (RTH258; brolucizumab): Novartis
- ADVM-022: Adverum Biotechnologies
- THR-149: Oxurion
- LKA651: Novartis
- And many others
Diabetic Macular Edema Market Report Scope
- The report covers the descriptive overview of DME, explaining its causes, signs and symptoms, pathophysiology, and currently available therapies.
- Comprehensive insight has been provided into the DME epidemiology and treatment in the 7MM.
- Additionally, an all-inclusive account of both the current and emerging therapies for DME is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape.
- A detailed review of DME market; historical and forecasted is included in the report, covering drug outreach in the 7MM.
- The report provides an edge while developing business strategies, by understanding trends shaping and driving the global DME market.
Request free sample copy- https://www.delveinsight.com/sample-request/diabetic-macular-edema-dme-market
Table of Content
1. Key Insights
2. Report Introduction
3. Diabetic Macular Edema Market Overview at a Glance
4. Executive Summary of Diabetic Macular Edema
5. Epidemiology and Market Forecast Flow
6. Disease Background and Overview
7. Current Treatment
8. Epidemiology and Patient Population
9. Patient Journey
10. Key Endpoints in Diabetic Macular Edema
11. Marketed Therapies
12. Emerging Therapies
13. Conjoint Analysis of DME Therapies
14. Diabetic Macular Edema: Seven Major Market Analysis
15. Market Access and Reimbursement
16. Market Drivers
17. Market Barriers
18. SWOT Analysis
19. Unmet Needs
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
23. About DelveInsight
Why should you buy this report?
- The report will help in developing business strategies by understanding trends shaping and driving the DME market.
- To understand the future market competition in the DME market and Insightful review of the key market drivers and barriers.
- Organize sales and marketing efforts by identifying the best opportunities for DME in the US, Europe (Germany, France, Italy, Spain, and the United Kingdom) and Japan.
- Identification of strong upcoming players in market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for DME market.
- To understand the future market competition in the DME market.
About Us
DelveInsight is a Business Consulting and Market research company, providing expert business solutions for life science vertical and offering quintessential advisory services in the areas of R&D, Strategy Formulation, Operations, Competitive Intelligence, Competitive Landscaping, and Mergers & Acquisitions.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email: Send Email
Phone: +919650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/