The ‘COVID-19 Novel Coronavirus 19 Therapeutic Pipeline Vaccines Diagnostics Competitive Landscape 2020’ report by DelveInsight offers a complete scenario of the R&D, development of therapies and diagnostic kits in the pipeline for COVID-19.
Novel coronavirus SARS-CoV-2 has wreaked havoc in the whole world with total confirmed cases of 2,721,354 worldwide. Heavy restrictions, lockdowns, quarantines, and social distancing are the only measures that have been proved to manage and contain the spread.
COVID-19 Diagnosis: Kits and tools in use and pipeline
As a precautionary measure, temperature screening of travellers has become a part of routine work. Anyone with signs and symptoms of COVID-19 such as fever, and shortness of breath, are tested for the disease. Present diagnostic kits include a healthcare provider collecting nasopharyngeal swabs from deep in the throat. However, recently Stanford has come up with a kit which tests the saliva of the patient without putting healthcare provider at risk of catching the infection.
Moreover, in an effort to provide efficient diagnosis, the FDA granted an Emergency Use Authorization to its first commercially developed coronavirus test developed by Roche (Cobas SARS-CoV-2 Test).
The report covers a comprehensive view of all the companies, institutions, and academics involved in the development of the diagnostic kits in use and in the pipeline for COVID-19.
COVID-19 treatment approaches and COVID-19 pipeline landscape
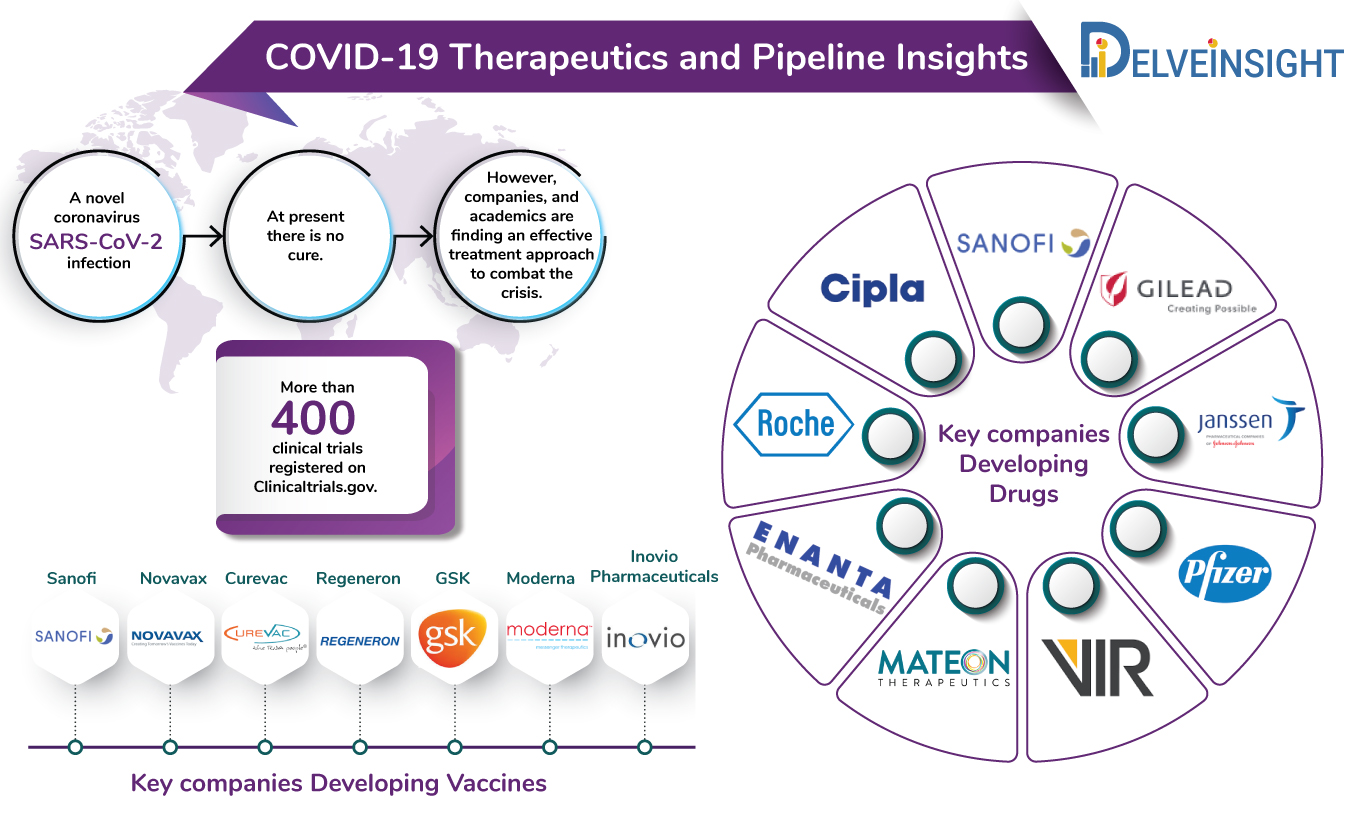
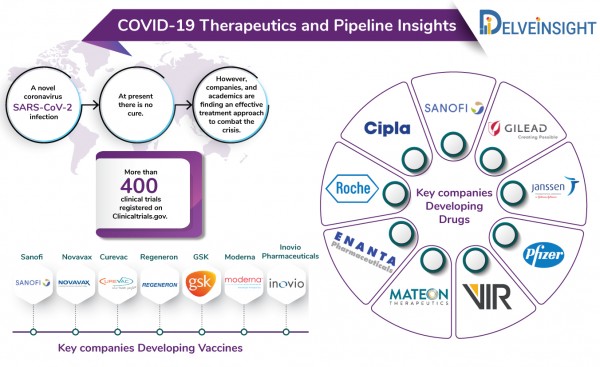
At the moment, there is no cure for coronavirus. However, efforts, all over the world, are underway to find a standard cure for coronavirus.
The report proffers insights into the present healthcare and pharma market scenario and shifted focus of the companies from their existing pipeline profile towards developing new treatment and preventive medicine against the novel SARS-CoV-2.
The current COVID-19 pipeline includes several big and small pharma companies including Gilead Sciences, Sanofi, Vir Biotechnologies, Regeneron, Cipla, Abbvie, Roche among others developing novel treatment approaches as well as repurposing their existing anti-inflammatory drugs to tackle the viral outbreak. Companies such as GSK, Takeda, Moderna and others are already running pre-clinical or advanced phases of trials for their vaccines against SARS-CoV-2.
Besides pharma companies, several healthcare institutions, hospitals, and universities including Columbian University, Stanford, Rutgers, Tongji Hospital, SouthWest Research Institute and others are aiding in developing the therapies to cure coronavirus infection.
The report offers a comparative clinical assessment of products by development stage, product type, route of administration, molecule type, and MOA type across this indication.
Scope of the report
- Better understanding of the technologies and techniques in use to develop -COVID 19 treatment drugs and vaccines.
- Comprehensive insights into COVID-19 pipeline products.
- Assessment of active pipeline assets segmented by stage, product type, route of administration and molecule type
- Key product-related agreements, collaborations, licensing, deals and technology in COVID-19 market.
- Overview of therapeutic assessment of the products by development stage, product type, route of administration, molecule type, and MOA type for -COVID 19 across the complete product development cycle, including all clinical and nonclinical stages.
- Key healthcare, and pharma companies and academics working in COVID-19 market
- Challenges and opportunities in the healthcare market due to COVID-19
- Detailed assessment of the diagnostic tests in development for of COVID 19 infection
Table of Contents
- Key Insights
- Coronavirus Disease 2019 (COVID 19) Overview at a Glance
- Disease Background and Overview: Coronavirus Disease 2019 (COVID 19)
- Diagnosis
- Current Treatment Practices
- COVID 19 – DelveInsight’s Analytical Perspective
- Current Trends
- Emerging Therapies
- Therapeutic Assessment
- Key Trends and Developments
- SWOT Analysis
- Market Drivers of COVID 19
- Market Barriers of COVID 19
- COVID 19 Unmet Needs
- Analyst Review
- Appendix
- DelveInsight Capabilities
- Disclaimer
About DelveInsight
DelveInsight is a premier Business Consulting and Market Research firm focused exclusively on the life science segment. With a wide array of smart end-to-end solutions, the firm helps the global Pharmaceutical and Bio-Tech companies formulate prudent business decisions for a better understanding of the pharma market.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Vinita Rakheja
Email: Send Email
Phone: 9193216187
Address:304 S. Jones Blvd #2432
Country: United States
Website: www.delveinsight.com/