InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global Chronic Total Occlusion Devices Market – (By Product Type (Microcatheters, Guidewires, Crossing Devices, Re-Entry Devices, Others), By End-Use (Hospitals & Diagnostic Center, Ambulatory Care Centers (ACC), Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031.”
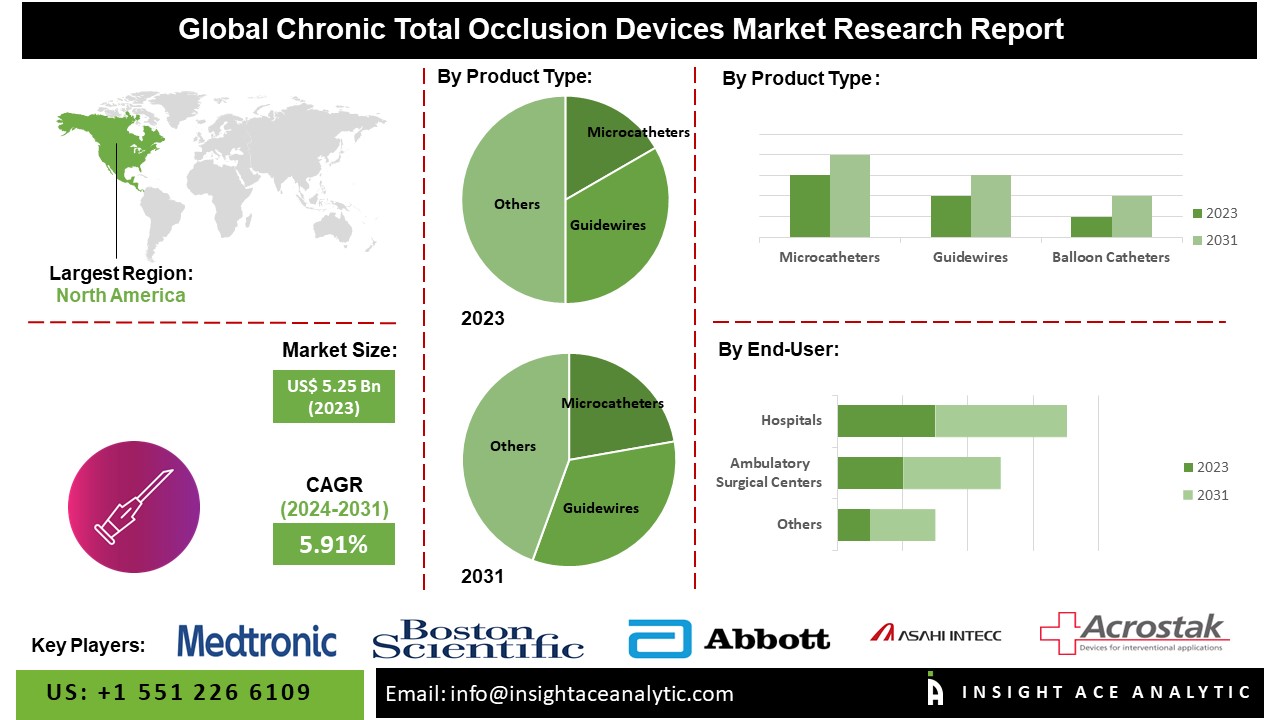
According to the latest research by InsightAce Analytic, the Global Chronic Total Occlusion Devices Market is valued at US$ 5.25 Bn in 2023, and it is expected to reach US$ 8.22 Bn by 2031, with a CAGR of 5.91% during the forecast period of 2024-2031.
Chronic total occlusion (CTO) devices are specialized medical instruments developed specifically for the management of chronic total occlusions (CTOs). These obstructions, which have been present in the coronary arteries for a minimum of three months, can be fully or nearly completely obstructed. Due to the existence of fibrous tissue and dense calcification, these obstructions are difficult to treat with conventional angioplasty and stenting methods. CTO pertains to complete blockages in coronary arteries, posing severe risks like heart attacks if left untreated. Consequently, there is a growing demand for CTO devices aimed at addressing these blockages effectively and enhancing patient outcomes. A primary catalyst for market expansion is the escalating prevalence of cardiovascular diseases globally, notably coronary artery disease. With the surge in such conditions, the necessity for advanced treatment options for chronic total occlusions becomes increasingly imperative.
Moreover, advancements in CTO device technology have led to the emergence of innovative products boasting enhanced efficacy and safety profiles. These devices offer minimally invasive treatment alternatives, thereby mitigating patient discomfort and accelerating recovery times compared to traditional surgical methods. Additionally, heightened awareness among healthcare professionals regarding the benefits of CTO devices and their rising integration into clinical practice are propelling market growth. Physicians are progressively acknowledging the significance of adeptly managing chronic total occlusions to bolster patient outcomes and mitigate complications.
Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/2396
List of Prominent Players in the Chronic Total Occlusion Devices Market:
- Medtronic
- Boston Scientific Corporation
- Terumo
- Cordis Corporation
- Spectranetics
- IntraLuminal Therapeutics
- Bard Peripheral Vascular
- Baylis Medical Company, Inc
- Soundbite Medical Solutions
- Acrostak Int. Distr. Sarl
- Abbott
- Asahi Intecc Co., Ltd.
- Cardinal Health
- Cook Medical
- Merit Medical Systems
- Koninklijke Philips N.V.
- Penumbra, Inc.
- Reflow Medical
- Teleflex Incorporated
- Becton, Dickinson and Company
- Others
Market Dynamics:
Drivers-
Healthcare providers are increasingly acknowledging the importance of effectively treating chronic total occlusions to enhance patient outcomes and mitigate the risk of complications. Consequently, there is a burgeoning focus on physician training programs, and educational initiatives centred on CTO interventions, fostering greater adoption of CTO devices and procedures. The escalation of healthcare expenditure, notably in emerging economies, plays a pivotal role in rendering CTO procedures and devices more affordable. As healthcare infrastructure advances and accessibility to specialized cardiac centres broadens, a larger number of patients gain entry to advanced CTO treatments, propelling market growth. Favourable reimbursement policies for CTO procedures and devices in specific regions, particularly in developed countries, incentivize healthcare providers to offer these interventions to eligible patients, thereby fostering the uptake of CTO devices and procedures and stimulating market expansion.
Challenges:
Despite progress, there persists a need for heightened awareness among patients as well as healthcare providers regarding the available treatment options for chronic total occlusions. Additionally, access to specialized centres equipped to perform CTO procedures may be restricted in certain regions, particularly in developing countries, thereby constraining market growth. The costliness of CTO procedures and devices renders them inaccessible to certain patient demographics, particularly those lacking adequate health insurance coverage or financial means. The substantial cost of treatment may serve as a deterrent to adoption and impede market expansion, particularly in regions with constrained healthcare budgets. Regulatory approval processes for CTO devices can be protracted and intricate, delaying their market entry and commercialization. Stringent regulatory requirements, notably in regions like North America and Europe, may pose challenges for manufacturers striving to introduce new CTO devices to the market, thereby limiting innovation and growth.
Regional Trends:
The North America Chronic Total Occlusion Devices market is poised to secure a significant market share in the CTO devices market, driven by its well-established healthcare infrastructure, high frequency of cardiovascular diseases, and early adoption of advanced medical technologies. However, the Asia Pacific region will may witness rapid market growth owing to improved healthcare infrastructure, escalating healthcare expenditure, and a burgeoning patient population. As the demand for effective treatment options for chronic total occlusions continues to surge, the market is primed for further expansion in the forthcoming years.
Curious About This Latest Version Of The Report? Enquiry Before Buying: https://www.insightaceanalytic.com/enquiry-before-buying/2396
Recent Developments:
- In May 2023, Reflow Medical, Inc. made an official declaration regarding the FDA commercial authorization it has obtained for its coraCatheters™, an extensive collection of cutting-edge microcatheters specifically designed for percutaneous coronary interventions to access and traverse intricate and demanding lesions. The most recent addition to Reflow’s portfolio of innovative devices is the coraCatheters family, which also comprises the Wingman™ and Spex™ Catheters. As the company establishes itself as an industry leader in coronary care, it is augmenting its sales force to provide assistance for present and forthcoming advancements.
- In Feb 2022, Teleflex Incorporated, a prominent worldwide supplier of medical technologies, received clearance from the U.S. Food & Drug Administration (FDA) to expand the use of its specialty catheters and coronary guidewires for crossing chronic total occlusion percutaneous coronary interventions (CTO PCI). CTOs refer to chronic total occlusions in coronary arteries, which are severe obstructions that completely obstruct the blood flow and cause a significant reduction in blood supply to the heart (ischemia).
Segmentation of Chronic Total Occlusion Devices Market-
By Product Type
- Microcatheters
- Guidewires
- Crossing Devices
- Re-Entry Devices
- Others
By End-Use
- Hospitals & Diagnostic Center
- Ambulatory Care Centers (ACC)
- Others
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- South East Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/2396
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/