InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global Cell and Gene Therapy Manufacturing Quality Control (QC) Market – (By Component (Equipment & Accessories, Consumables, Others), By Application (Sterility Testing, Purity Testing, Potency Testing, Identity Testing, Others (stability, viability, etc.)), By Process (Upstream Processing, Downstream Processing, Packaging), By End-User (Pharmaceutical & Biotechnology Companies, Contract Manufacturing Organizations)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031.”
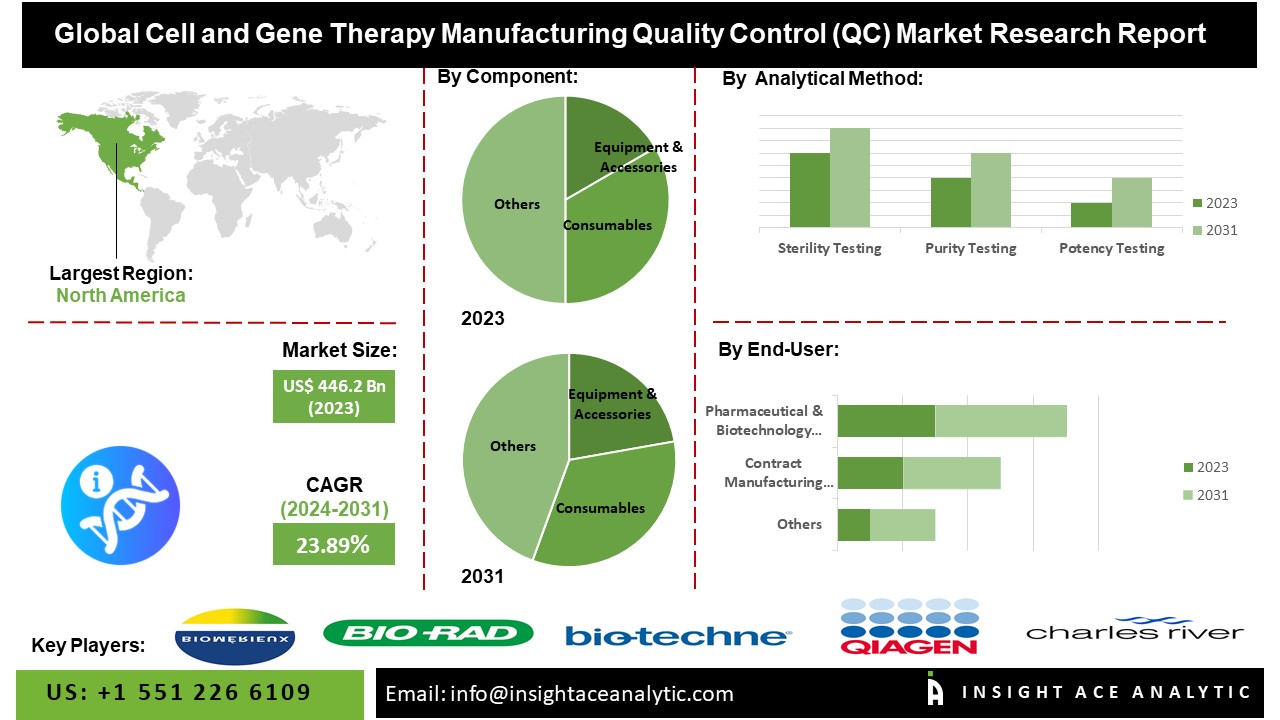
According to the latest research by InsightAce Analytic, the Global Cell and Gene Therapy Manufacturing Quality Control (QC) Market is valued at US$ 446.2 Mn in 2023, and it is expected to reach US$ 2,434.4 Mn by 2031, with a CAGR of 23.89% during the forecast period of 2024-2031.
The cell & gene therapy manufacturing quality control (QC) market encompasses a range of processes and procedures aimed at ensuring the safety, efficacy, and consistency of cell and gene therapy products. With the rapid advancement of cell and gene therapy technologies, there is an increasing emphasis on quality control measures to match regulatory requirements and deliver high-quality therapeutic products to patients worldwide. Regulatory agencies worldwide are increasingly approving cell and gene therapy products for clinical use, leading to a growing market for manufacturing and quality control services. Moreover, the approval of new therapies necessitates robust quality control measures to ensure compliance with regulatory standards and patient safety. Pharmaceutical companies, biotechnology firms, and academic institutions are investing heavily in the research and development of cell and gene therapies. These investments aim to develop novel therapies, improve manufacturing processes, and enhance quality control measures to meet regulatory requirements and market demand. Additionally, governments and funding agencies are providing support and incentives for the development and commercialization of cell and gene therapies. Funding programs, grants, and tax incentives encourage companies to invest in manufacturing and quality control infrastructure, driving market growth.
Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/2395
List of Major Players in the Cell and Gene Therapy Manufacturing Quality Control (QC):
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- QIAGEN
- Charles River Laboratories International, Inc.
- Lonza Group AG
- Merck KGaA
- Intertek Group plc
- Thermo Fisher Scientific, Inc.
- Eurofins Scientific S.E.
- F. Hoffmann-La Roche Ltd.
- Catalent
- Wuxi AppTec
- Takara Bio Inc
- Oxford Biomedica plc
- Cell and Gene Therapy Catapult
- FUJIFILM Holdings Corporation
- Danaher (Cytiva)
- Sartorius AG
- AGC Biologics.
- Eurofins Scientific
- Other Market Players
Market Dynamics:
Drivers-
The Cell & Gene Therapy Manufacturing Quality Control (QC) market is boosted by the multiplying demand for personalized medicine, advancements in cell and gene therapy technologies, increasing investments in research and development, and expanding regulatory approvals for cell and gene therapy products—additionally, the rising occurrence of chronic diseases and genetic disorders further fuels market growth. Continuous advancements in cell and gene therapy technologies have led to the development of innovative therapies for various diseases, driving the demand for quality control measures. As the field progresses, there is a growing need for stringent quality control processes to ensure the safety, efficacy, and consistency of therapeutic products.
Challenges:
The Cell & Gene Therapy Manufacturing Quality Control (QC) market encounters several significant challenges. These include intricate manufacturing processes, stringent regulatory demands, elevated production expenses, and technical and logistical hurdles related to scaling up production to meet market demands. Additionally, ensuring the consistency and reproducibility of cell and gene therapy products poses continual challenges for manufacturers.
Regional Trends:
The North America Cell and Gene Therapy Manufacturing Quality Control (QC) market is expected to lead the global market revenue share. Factors such as well-established healthcare infrastructure, significant investments in research & development, the presence of key market players, and a supportive regulatory environment contribute to the region’s market leadership. Europe holds a considerable share in the Cell and Gene Therapy Manufacturing Quality Control (QC) market, driven by the increasing adoption of innovative therapies, favourable government initiatives, and the robust pharmaceutical industry. The region is characterized by growing collaborations between industry stakeholders and academic institutions, fostering innovation and market growth.
Curious About This Latest Version Of The Report? Enquiry Before Buying: https://www.insightaceanalytic.com/enquiry-before-buying/2395
Recent Developments
- In Feb 2024, Thermo Fisher Scientific has established a novel sterile drug facility in Singapore, which will enhance the company’s capacity to supply clients in the Asia-Pacific region with novel pharmaceuticals and vaccines. In addition to being an investment in pandemic preparedness, the new facility signifies a significant achievement and milestone for Singapore, which is rapidly becoming a biomedical centre in the Asia-Pacific region.
- In Apr 2020, Merck, a prominent scientific and technological business, has declared the creation of a second establishment in Carlsbad, California, USA, specifically for its BioReliance® viral and gene therapy services. The anticipated inauguration of the state-of-the-art commercial establishment, with a budget of €100 million, is scheduled for the fiscal year 2021-2022.
Segmentation of Cell and Gene Therapy Manufacturing Quality Control (QC)-
By Component
- Equipment & Accessories
- Consumables
- Others
By Application
- Sterility Testing
- Purity Testing
- Potency Testing
- Identity Testing
- Others (stability, viability, etc.)
By Process-
- Upstream Processing
- Downstream Processing
- Packaging
By End-User-
- Pharmaceutical & Biotechnology Companies
- Contract Manufacturing Organizations
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/2395
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/