While various techniques have been developed to address the safety issues in lithium-ion batteries, there remain unknown mechanisms that induce hazards, leaving challenges in developing reliable lithium-ion systems. In this manuscript, an unpredictable hazard originated from the dissolution of transition metal ions from cathodes and their deposition on anodes is presented. The cathodes in currently commercialized lithium-ion batteries contain transition metal ions, which might dissolve in electrolytes, transport through separators and deposit on anodes. When the deposition proceeds in the form of metals, the deposited transition metals might induce a hazard due to the lithium dendrite growth on them. Since the electrolyte decomposition usually accompanies the transition metal deposition with a possibility that the electrolyte decomposition products might cover the deposited metals, the induced hazard becomes unpredictable.
In currently commercialized lithium-ion batteries, cathodes contain transition metal ions, such as iron ion in LiFePO4, cobalt ion in LiCoO2, manganese ion in LiMn2O4, and nickel, cobalt and manganese ions in LiNixCoyMnzO2 (x + y + z = 1). The dissolution of transition metal ions from cathodes into electrolytes is inevitable during charge/discharge process or storage of lithium ion batteries, especially under elevated temperature and cell voltage. The dissolved transition metal ions (Mn+) might presents various effects on battery performances. Especially, Mn+ might transport through separators and deposit on anodes, as shown in Fig. 1. Even in the cycled lithium-ion battery based on LiFePO4 cathode, which has stable crystal structure and works under low voltage, large amount of iron can be detected on the graphite anode from used cell. If the deposition proceeds in a reduction process and transition metals (M in Fig. 1) are present on anodes, the lithium dendrite growth will take place on the transition metals, resulting in an internal short circuit and inducing a hazard of lithium-ion batteries. Since the electrolyte decomposition usually accompanies the transition metal deposition, the electrolyte decomposition products might cover the deposited transition metals, which make the induced hazard become unpredictable: when and where the deposited transition metals are available for lithium dendrite growth. To avoid this unpredictable hazard, it is necessary to eliminate the dissolution of transition metal ions and their deposition on anodes.
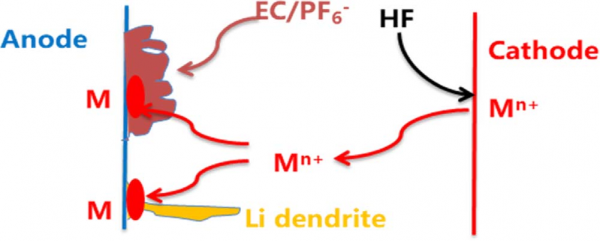
Figure 1. Schematic illustration of the dissolution of transition metal ions from cathode, their deposition as metals on anode and unpredictable lithium dendrite growth on metals.
There have been various strategies available for suppressing the dissolution of transition metal ions from cathodes of lithium-ion batteries and their deposition on anodes. To address the issue of transition metal ion dissolution, the fifirst consideration is to stabilize the structure of cathode materials. This consideration includes doping other elements into crystal lattices, developing nano-particles, constructing porous structures,10,38–41 and growing typical crystal planes. Similarly, binding material particles with polymers can also improve the structure stability of cathodes. No matter how to stabilize the structure of the cathode materials, the transition metal ions might dissolve from cathode materials, because the attack of HF from electrolyte is inevitable.
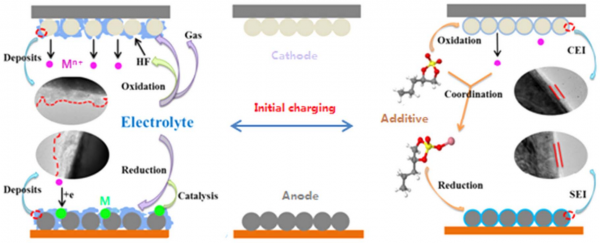
The transition metal ions that are present in the cathodes of lithium-ion batteries might dissolve from cathodes into electrolytes, transport through separators and deposit on anodes. When the deposition of the transition metal ions takes place in the form of metals, the deposited transition metals provide active sites for lithium dendrite growth, yielding a hazard of lithium-ion batteries. Since the deposition of transition metal ions is accompanied by the electrolyte decomposition, the resulting electrolyte decomposition products might cover the deposited metals and this hazard is unpredictable. Therefore, it is necessary to eliminate the dissolution of transition metal ions from cathodes or their deposition on anodes in the form of metals. The first consideration is to fabricate the cathodes with stable crystal structures. The dissolution of transition metal ions is related to the presence of HF in the electrolytes, which results mainly from thermal and electrochemically oxidative decom position of the salt LiPF6 in the electrolytes.
Therefore, a number of strategies have been developed to separate cathodes from direct contact with electrolyte or inhibit the formation of HF. For separation, the strategies can be to coat cathodes with inert compounds and to construct protective cathode interphase fifilms with electrolyte additives. For inhibition, salts that are more stable than LiPF6 and the additives that can coordinate with HF or other species from the decomposition of LiPF6 could be effificient. On the other hand, the deposition of transition metal ions on anodes in the form of metals could be avoided by coordinating transition metal ions with electrolyte additives and isolating transition metal ions from anodes with modifified separators that allow the transportation of lithium ions but prevent the penetration of transition metal ions.
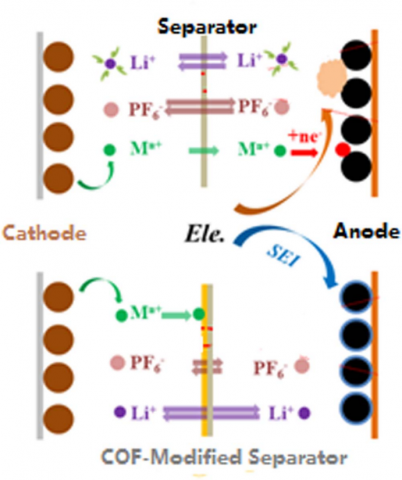
Figure 3. The separator that can regulate the transportation of lithium ion and transition metal ions.
In the future, JUNLEE Energy lithium battery manufacturer, which has been committed to battery research and development, is a challenge and an opportunity. The R&D team of engineers will provide the world with more economical new energy batteries, and will improve lithium-ion battery technology to reduce the total cost.
JUNLEE Group is an integrated full power energy factory that specializes in Uninterruptible Power Supply (UPS), Lead-Acid Battery, Battery pack, EV battery, Energy Storage Battery, Energy storage power station, Power pack Gel battery, PV Inverter and Solar system.
Production capacity reach 200000 KVaH per month. Products apply to Electric vehicles, electric mobility, solar & wind energy storage system, UPS, backup power, telecommunication, medical equipment and lighting.
JUNLEE sets up “Power research center” with more High-tech products. More than 100 engineers provided in-time and efficient one-stop solutions.
They mission strives to bring green power to the world.
To learn more about Li-ion batteries, please refer to https://www.junleepower.com/
Media Contact
Company Name: JUNLEE Energy
Contact Person: Media Relations
Email: Send Email
Phone: +(86) 13434236097
Address:Building 1, No.3, Yanshanwei Lane 1, Tangxia Town
City: Dongguan
State: Guangdong
Country: China
Website: https://www.junleepower.com/
