DelveInsight launched a new report on Acromegaly Pipeline Insight, 2020.
“Acromegaly Pipeline Insight, 2020” report by DelveInsight outlays comprehensive insights of present clinical development scenario and growth prospects across the Acromegaly market. A detailed picture of the Acromegaly pipeline landscape is provided, which includes the disease overview and Acromegaly treatment guidelines.
The assessment part of the report embraces in-depth Acromegaly commercial assessment and clinical assessment of the Acromegaly pipeline products from the pre-clinical developmental phase to the marketed phase. In the report, a detailed description of the drug is proffered including mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, Acromegaly collaborations, licensing, mergers and acquisition, funding, designations, and other product-related details.
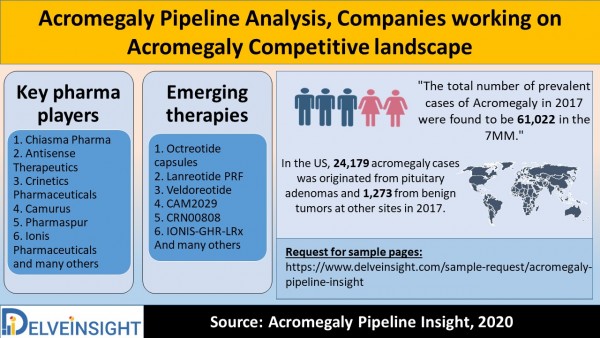
“The total number of prevalent cases of Acromegaly in 2017 were found to be 61,022 in the 7MM.”
The key players in Acromegaly market are:
1. Chiasma Pharma
2. Antisense Therapeutics
3. Crinetics Pharmaceuticals
4. Camurus
5. Pharmaspur
6. Ionis Pharmaceuticals
7. Strongbridge Biopharma
8. Midatech
9. Ipsen
10. Dauntless Pharmaceuticals
11. Ono Pharmaceuticals
And many others
The launch of the emerging therapies is expected to significantly impact the Acromegaly treatment scenario in the upcoming years:-
Drugs covered
1. Octreotide capsules
2. Lanreotide PRF
3. Veldoreotide
4. CAM2029
5. CRN00808
6. IONIS-GHR-LRx
And many others
Request for sample pages: https://www.delveinsight.com/sample-request/acromegaly-pipeline-insight
Pipeline Development Activities: ACROMEGALY
The report provides insights into:
1. All of the companies that are developing therapies for the treatment of Acromegaly with aggregate therapies developed by each company for the same.
2. Different therapeutic candidates segmented into early-stage, mid-stage and late stage of development for the Acromegaly treatment.
3. Acromegaly key players involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
4. Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
5. Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of Acromegaly market.
The report is built using data and information traced from the researcher’s proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations, and featured press releases from company/university web sites and industry-specific third-party sources, etc.
Scope of the report:
1. The Acromegaly report provides an overview of therapeutic pipeline activity and therapeutic assessment of the products by development stage, product type, route of administration, molecule type, and MOA type for Acromegaly across the complete product development cycle, including all clinical and nonclinical stages.
2. It comprises of detailed profiles of Acromegaly therapeutic products with key coverage of developmental activities, including technology, collaborations, licensing, mergers and acquisition, funding, designations and other product-related details
3. Detailed Acromegaly research and development progress and trial details, results wherever available, are also included in the pipeline study.
4. Coverage of dormant and discontinued pipeline projects along with the reasons if available across Acromegaly.
Report highlights:
1. A better understanding of disease pathogenesis contributing to the development of novel therapeutics for Acromegaly.
2. In the coming years, the Acromegaly market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market.
3. The companies and academics that are working to assess challenges and seek opportunities that could influence Acromegaly R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition.
4. A detailed portfolio of major pharma players who are involved in fueling the Acromegaly treatment market. Several potential therapies for Acromegaly are under investigation. With the expected launch of these emerging therapies, it is expected that there will be a significant impact on the Acromegaly market size in the coming years.
5. Our in-depth analysis of the pipeline assets (in early-stage, mid-stage and late stage of development for the treatment of Acromegaly) includes therapeutic assessment and comparative analysis. This will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities.
Table of contents:
1. Report Introduction
2. Acromegaly
3. Acromegaly Current Treatment Patterns
4. Acromegaly – DelveInsight’s Analytical Perspective
5. Therapeutic Assessment
6. Acromegaly Late Stage Products (Phase-III)
7. Acromegaly Mid Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Acromegaly Discontinued Products
13. Acromegaly Product Profiles
14. Acromegaly Key Companies
15. Acromegaly Key Products
16. Dormant and Discontinued Products
17. Acromegaly Unmet Needs
18. Acromegaly Future Perspectives
19. Acromegaly Analyst Review
20. Appendix
21. Report Methodology
Download a full report: https://www.delveinsight.com/sample-request/acromegaly-pipeline-insight
Related reports:
Acromegaly – Global API Manufacturers, Marketed and Phase III Drugs Landscape, 2020
Acromegaly – Epidemiology Forecast to 2030
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email: Send Email
Phone: 9193216187
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/

