The global breakthrough therapy designation market is anticipated to reach USD 144.6 billion by 2025, according to a new report by Grand View Research, Inc. Breakthrough Therapy (BT) designation is granted to drugs that display substantial results in the treatment of life-threatening diseases in initial stages of the drug development process. Expedited regulatory process in North America and EU regions is driving growth. Increase in the number of innovative molecules receiving the BT status, coupled with rise in the demand for orphan drugs as well as those for the treatment of cancer, is anticipated to boost the market over the forecast period.
The development time of BT therapy is 30.0% to 50.0% lesser than those without the designation. This significantly increases the net present value of investigational molecule. Reduction in the development time of these drugs is expected to accelerate their approval process as compared to standard drugs as well as boost growth opportunities. The BT tag also increases the value of the drug due to its high scientific value.
There has been a consistent rise in the number of drugs receiving BT status in the U.S., and this trend is also expected to drive the number of applications submitted for the same, thereby contributing to the market growth.
Full Research Report On BT Designation Market Analysis: https://www.grandviewresearch.com/industry-analysis/breakthrough-therapy-bt-designation-market
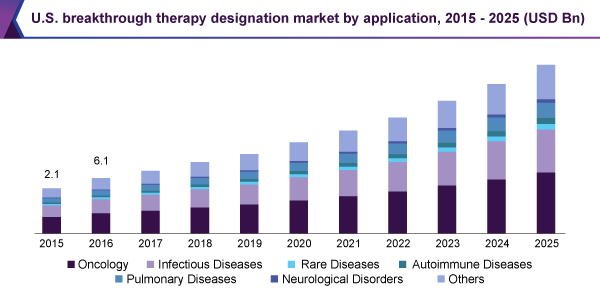
Further Key Findings From the Study Suggest:
- The infectious diseases segment dominated the market in 2016. However, it is expected to exhibit moderate growth in the forecast period owing to decline in sales of blockbuster drugs such as Harvoni and Sovaldi
- Oncology is expected to be the dominating segment over the forecast period owing to rise in the demand for cancer medicines and increase in the number of oncology molecules gaining BT status. As of 2017, the number of molecules gaining BT status is the highest in this segment
- The rare diseases segment is estimated to exhibit fastest growth owing to rise in demand and increase in the R&D pipeline of orphan drugs
- North America is expected to dominate the market over the forecast period owing to well-established intellectual property laws and preference of patients and healthcare professionals for innovative medicines
- Some of the key players are F. Hoffmann-La Roche Ltd; Gilead; Novartis AG; Pfizer, Inc.; AbbVie, Inc.; Janssen Global Services, LLC; Bristol-Myers Squibb Company; Eli Lilly and Company; Sanofi; Regeneron; AcadiaPharmaceuticals, Inc.; Boehringer Ingelheim GmbH; Amgen, Inc.; AstraZeneca; and GlaxoSmithKline plc.
View More Reports Of This Category By Grand View Research At: https://www.grandviewresearch.com/industry/pharmaceuticals
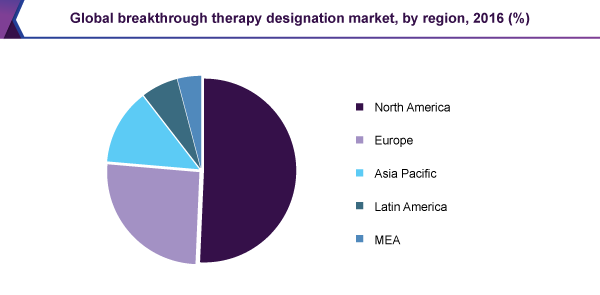
Grand View Research has segmented the breakthrough therapy market report on the basis of application and region.
Breakthrough Therapy Designation Application Outlook (Revenue, USD Million, 2015 – 2025)
- Oncology
- Infectious Diseases
- Rare Diseases
- Auto Immune Diseases
- Pulmonary Diseases
- Neurological Disorder
- Others
Breakthrough Therapy Designation Regional Outlook (Revenue, USD Million, 2015 – 2025)
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- Asia Pacific
- Japan
- China
- Latin America
- Brazil
- Mexico
- Middle East and Africa (MEA)
- South Africa
- Saudi Arabia
Access Full Press Release Of This Report By Grand View Research: https://www.grandviewresearch.com/press-release/global-breakthrough-therapy-bt-designation-market
About Grand View Research
Grand View Research, Inc. is a U.S. based market research and consulting company, registered in the State of California and headquartered in San Francisco. The company provides syndicated research reports, customized research reports, and consulting services. To help clients make informed business decisions, we offer market intelligence studies ensuring relevant and fact-based research across a range of industries, from technology to chemicals, materials and healthcare.
For more information: www.grandviewresearch.com
Media Contact
Company Name: Grand View Research, Inc.
Contact Person: Sherry James, Corporate Sales Specialist – U.S.A.
Email: sales@grandviewresearch.com
Phone: 1-415-349-0058, Toll Free: 1-888-202-9519
Address:28 2nd Street, Suite 3036
City: San Francisco
State: California
Country: United States
Website: www.grandviewresearch.com/industry-analysis/breakthrough-therapy-bt-designation-market

